
Immortalized Rat Retinal Müller Cells (rMC-1)
Cat.No.: CSC-I9199L
Species: Rattus norvegicus
Source: Retina
Morphology: Large, flat cells
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
2) Immunocytochemistry was used to confirm the nuclear expression of SV40-LTA in the immortalized cells.
The Immortalized Rat Retinal Müller Cell line (rMC‑1) is a popular in vitro model of retinal glial physiology. Derived from the retina of Sprague‑Dawley rats via SV40‑mediated immortalization, rMC‑1 expresses classic Müller‑cell markers including GFAP, CRALBP, vimentin, and glutamine synthetase to confirm lineage fidelity. In culture, the cells take on a flat, epithelial‑like morphology and grow as a confluent monolayer on typical tissue‑culture plastic, reaching 80-90 % confluency in 24 h after entering the logarithmic growth phase. Cells expand vigorously in high‑glucose DMEM media with 10 % fetal bovine serum, penicillin/streptomycin, and L‑glutamine, and can be passaged at a 1:2-1:3 ratio using low‑concentration trypsin.
Researchers can use rMC‑1 to study diabetic retinopathy alongside oxidative stress and inflammatory pathways because this cell line expresses essential Müller‑cell functions like potassium buffering and glutamate uptake while demonstrating antioxidant potential. The cell line has been used in drug‑screening assays, gene‑silencing experiments, CRISPR editing, and co‑culture systems to study processes such as neurovascular coupling and blood‑retinal barrier formation.
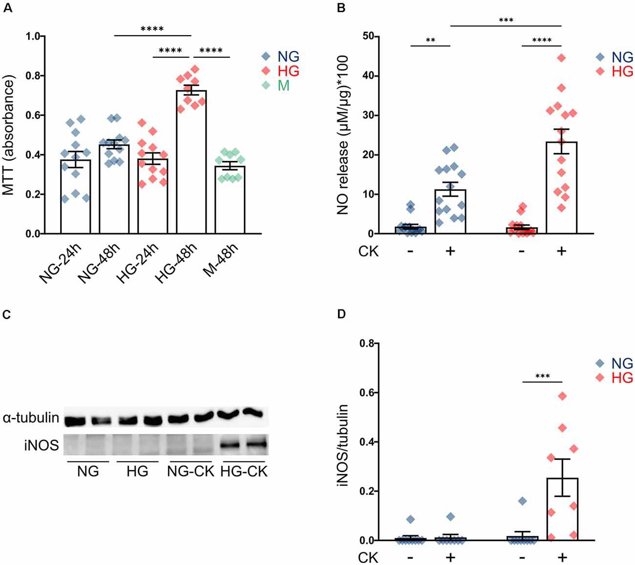
Effect of High Glucose on Müller Cell Line (rMC-1) Proliferation and Activation
Diabetic retinopathy (DR) is a prevalent microvascular complication of diabetes mellitus and the leading cause of vision loss. Although classically considered a microvascular disease, growing evidence is showing that neurodegeneration can be an early event in DR before the development of vasculopathy. Rosato et al. investigated functional changes in Müller cells (rMC-1), the most abundant retinal glial cells, under high glucose conditions mimicking DR, focusing on oxidative stress and inflammatory responses.
rMC-1 cells were cultured in normal glucose (5 mM; NG) or high glucose (35 mM; HG) for 24-48 hours. Cell proliferation was assessed using the MTT assay. Cells cultured in NG did not show any change in growth rate, while cells in HG for 48 hours had significantly higher proliferation compared to HG for 24 hours or NG (Fig. 1A). Mannitol (35 mM) did not have any effect on proliferation. These data were consistent with in vivo Müller cells under diabetic conditions. In diabetic rats, chronic exposure to HG induced proliferation through activation of proliferative pathways. To investigate if HG activates rMC-1 cells or amplifies pro-inflammatory effects, cells were cultured in NG or HG for 48 hours and treated with a cytokine mix (rat IL-1β, human IFN-γ, human TNF-α) for the last 24 hours. NO release and iNOS expression were used as activation markers. HG alone did not induce NO release (Fig. 1B, HG untreated), but CK treatment did, and this was enhanced with HG (Fig. 1B). iNOS expression was only detected in HG-treated cells with CKs (Fig. 1C, D). These results suggest that HG can exacerbate the activation response of Müller cells.
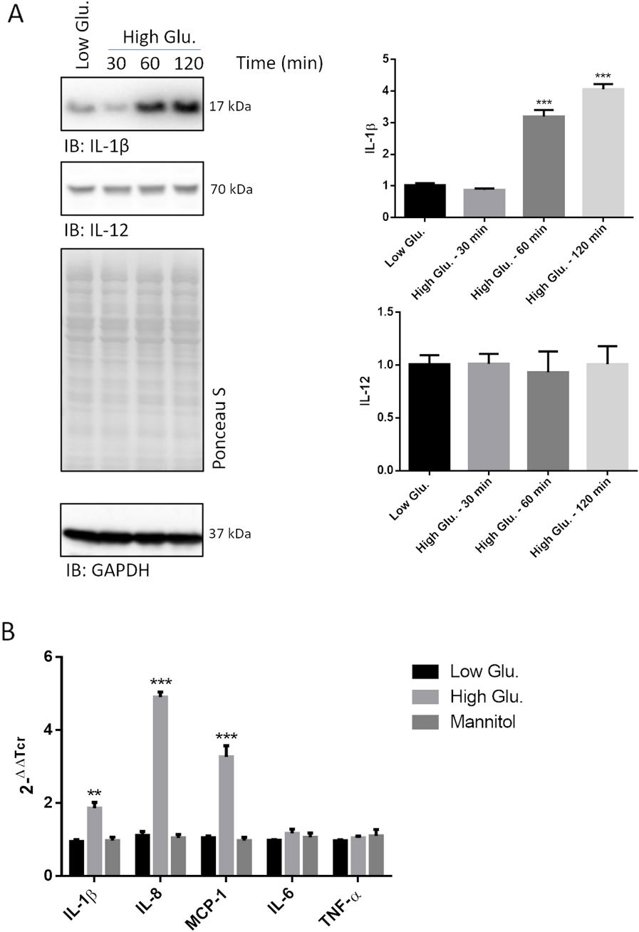
Transcription of Pro-Inflammatory Cytokines is Induced Early by High Glucose Concentrations in rMC1
Diabetic retinopathy (DR) is a microvascular complication of diabetes that significantly impacts quality of life and imposes a substantial burden on health and economic systems. The role of Müller glia (MG) in sensing hyperglycemia and acquiring pro-inflammatory polarization is highlighted in DR pathogenesis. Using an in vitro DR model with rMC1 cells, Sbardella et al. investigated how high glucose induces early NF-kB signaling.
They observed that the 17 kDa IL-1β fragment (the active form) was highly expressed in rMC1 cells treated with 25 mM glucose (high glucose) for up to 2 hours (Fig. 2A). Cells treated with 5 mM glucose or mannitol (controls) showed much lower cytokine expression. IL-12 and IL-10 levels remained unchanged. To check if the IL-1β increase was due to transcriptional upregulation, they performed RT-PCR on rMC1 cells treated with 25 mM glucose for 40 minutes. High glucose treatment led to a ~twofold increase in IL-1β mRNA (Fig. 2B). Other cytokines linked to NF-kB activation were also tested: IL-8 (~fivefold increase) and MCP-1 (~threefold increase) were highly induced, while IL-6 and TNFα were not (Fig. 2B).
Ask a Question
Write your own review
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells

