Primary Cell Culture Guide
For decades, cell lines have played a critical role in scientific developments. In most cases, researchers just got data generated from cell lines. However, due to some weaknesses of cell lines, scientists become increasingly cautious about these generated results. But now the game has changed! Primary cells now are believed to be a more biologically relevant tool than cell lines for studying human and animal biology. And we design this primary cell culture guide aimed at showing new investigators the basic principles of primary cell and some practical culture skills.
Basic Properties of Primary Cells
Primary cells are cells isolated directly from human or animal tissue by using enzymatic or mechanical methods. After isolation, they are placed in an artificial environment and established for growth. According to the genus (by species or tissues) from which they are isolated, they can be categorized in epithelial cells, fibroblasts, keratinocytes,melanocytes, endothelial cells, muscle cells, hematopoietic and mesenchymal stem cells.
Primary cells differ from cell lines in many ways.
- Primary cells have a finite lifespan. After a certain period of time in culture, they will die. And the amount of time primary cells survives in culture varies depending on the cell type. On the contrary, an immortalized or continuous cell line has acquired the ability to proliferate indefinitely.
- Primary cell cultures are harsh, requiring optimized growth conditions, including the addition of specific cytokines and growth factors for propagation in serum-free or low-serum growth media, which is absolutely different from immortalized cell lines.
- Primary cells retain the natural feature of the tissue where they had been isolated. And due to this character, they are physiologically normal and thus theoretically provide unaltered, “natural” experimental results. Immortalized cell lines, on the other hand, are often transformed with other inducible modifications, which may alter the outcome of experiments.
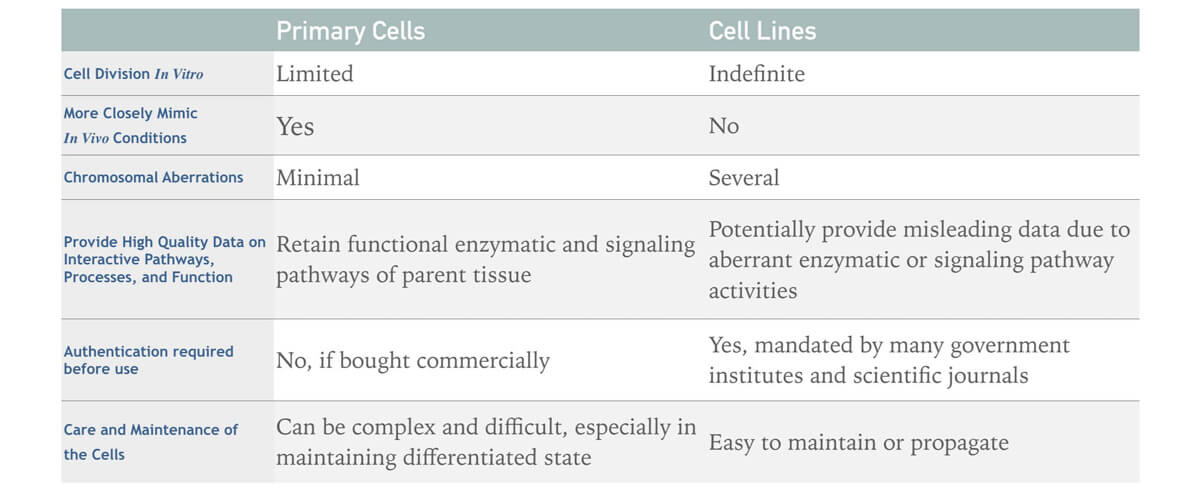
Table 1. The list of major differences between primary cells and cell lines.
Benefits of Primary Cells
Generally, primary cells retain normal morphology, cellular function, growth characteristics, cellular markers, signaling and genetic integrity when propagated in culture. That's why they are commonly used as in vitro tools for pre-clinical and investigative biological research. Meanwhile, primary cells from different species may be used which can highlight potential differences between humans and preclinical test species. And before in vivo studies, mouse or rat primary cells can be used to refine doses and reduce the number of animals required for preclinical toxicology for cost-saving. In addition, the use of primary cells containing none mutations and chromosomal abnormalities has served as the best representative indicators of normal cell phenotype and progression of the early-stage disease. They also can be used to determine the accuracy of extrapolating human data from an animal model.
The General Process of Primary Cells Culture
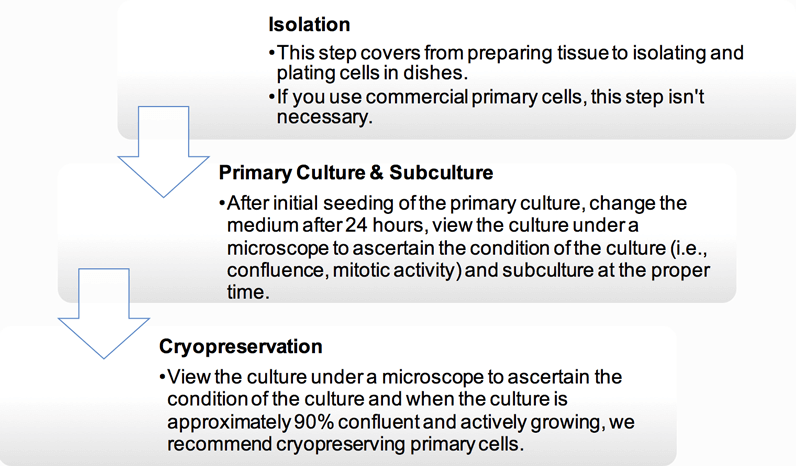
Isolation of Primary Cells
The isolation and purification of peripheral blood cells can be easily achieved by differential centrifugation or by positive sorting using magnetic beads. But the isolation of a pure population of cells from tissue is always difficult to perform, and it is necessary to apart into a suspension containing only one predominant cell type. And Figure 1 is an illustration of some of the basic steps used to establish a primary cell culture. Different kinds of primary cells have their own methods to be isolated from primary tissue, we provide the detailed protocol in technical bulletin.
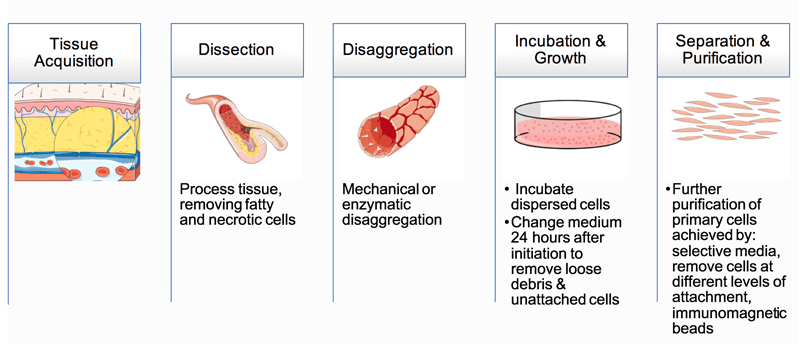 Figure 1. Basic steps used to isolate cells from primary tissue.
Figure 1. Basic steps used to isolate cells from primary tissue.
Primary Cell Culture
- Growth Requirements
There are two categories of primary cells – adherent or suspension. Suspension cells, derived from peripheral blood without the requirement of attachment for growth, are said to be anchorage-independent cells. On the other hand, adherent primary cells require a surface such as a flat un-coated plastic vessel in order to grow properly in vitro. No matter which type the primary cells are, a complete cell culture media with appropriate growth factors and cytokines is required.
Strict aseptic operation from cell isolation to cell storage can prevent cell contamination. During the establishment of primary cultures, it may be useful to include an antibiotic in the growth medium to inhibit contamination introduced from the host tissue. But long-term use of antibiotics may be toxicity to cells over time which is not advised.
- Maintenance & Subculture
The maintenance phase will begin once cells attached to the surface of the culture dish. About 24 hours after initiation of the culture, the spent media should be removed and the condition of the culture must be viewed in order to determine the proper time to subculture. When cells have reached a desired percent of cellular confluence, that is to say, they are actively proliferating and it is a proper time to subculture.
The level of cellular confluency is important as the cells change their growth with changing densities. Low-density cells (10-20%) usually grow slower than 50% confluent cells. If the plate is completely grown by cells, they will tend to grow much slower again. And the changes of growth rate will influence their genetic program, behavior in experiments and transfection efficiency. So the best cellular confluency rate is approximately 90%.
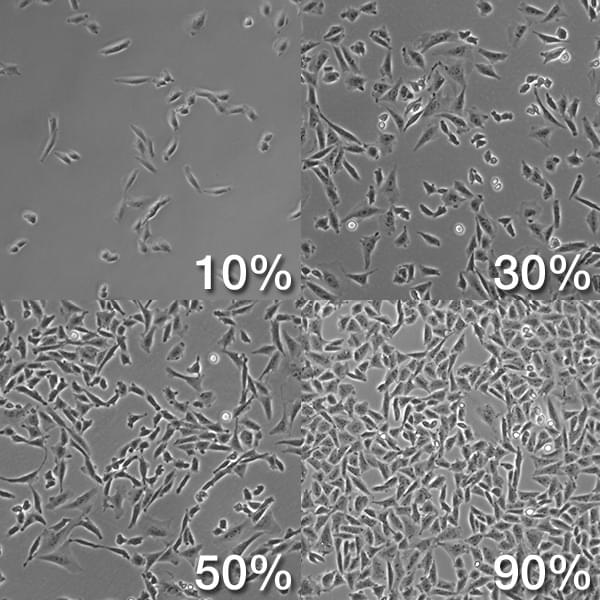 Figure 2. The level of cellular confluency.
Figure 2. The level of cellular confluency.
See the technical bulletin for more culture information of specific primary cell types.
Cryopreservation and Recovery
The method of cryopreserving and thawing primary cells is similar to cell lines, special attention needed is minimized cell damage and death during each process.
Most primary cell cultures can be cryopreserved in a mixture of 80% complete growth medium supplemented with 10% FBS and 10% DMSO. The freezing process should be slow to prevent the formation of ice crystals within the cells. And frozen cultures should be stored in the vapor phase of liquid nitrogen, or below -130°C. Important attention needed: in order to get a reliable and veritable result with primary cells, the ideal generations should be 2-10. It's better to conserve cultures in time.
Thawing cryopreserved cells is a rapid process. Special care should be taken not to centrifuge primary cells upon thaw as they are extremely sensitive to damage during recovery from cryopreservation. It is best to plate cells directly upon thaw, and maintain first 24 hours before changing the medium.
Getting Started with Creative Bioarray
Creative Bioarray provides a comprehensive solution to help our clients overcome the high cost and inconsistency found in routine primary cell culture. A highly-standardized cell culture system that includes high-quality cells, medium, supplements, reagents and protocols. Our primary cell solutions have focused on providing researchers with superior quality commercial primary cells from a trusted source - each lot of primary cells is:
- Cryopreserved at early passage 1 through 3
- Higher viability and optimal plating efficiency performance tested
- Optimal reliable cells, media, kit supplements, and reagents
Creative Bioarray is a global provider of comprehensive cell culture solutions for the bioscience industry. We provide a portfolio of flexible and integrated solutions for cell culture including biobank, cell isolation, cell authentication and custom culture, cold-chain cryogenic storage systems, and global cold-chain logistics. Please feel free to contact us for a further discussion.
References
- Mather J P, Barnes D W. Animal cell culture methods[M]. Academic Press, 1998.
- Worthington Biochemical Corporation. Cell isolation theory & Tissue Dissociation Guide[M]. 2000.
- Mattinger C, Nyugen T,; et al. Evaluation of serum-free culture conditions for primary Mouse nasal epithelial cells. Int J Hyg Environ Health. 2002,205(3):235-8.
- Koller, M.R.P., B. O.; Masters, J. R. W. Human Cell Culture: Primary Hematopoietic Cells[M]. Springer, 1999.