Ocular Toxicity
- Service Details
- Features
- FAQ
- Explore Other Options
Ocular toxicity is the potential of a chemical or therapeutic agent to cause adverse effects on the structure or function of the eye. The unique anatomy and physiology of the eye make it particularly vulnerable to toxic effects, even at low doses, which can lead to significant vision loss or permanent damage. Regulatory agencies such as the FDA, EMA, and OECD have highlighted the importance of ocular safety evaluation, particularly for compounds that are intended for systemic administration, ophthalmic use, or with potential for occupational exposure.
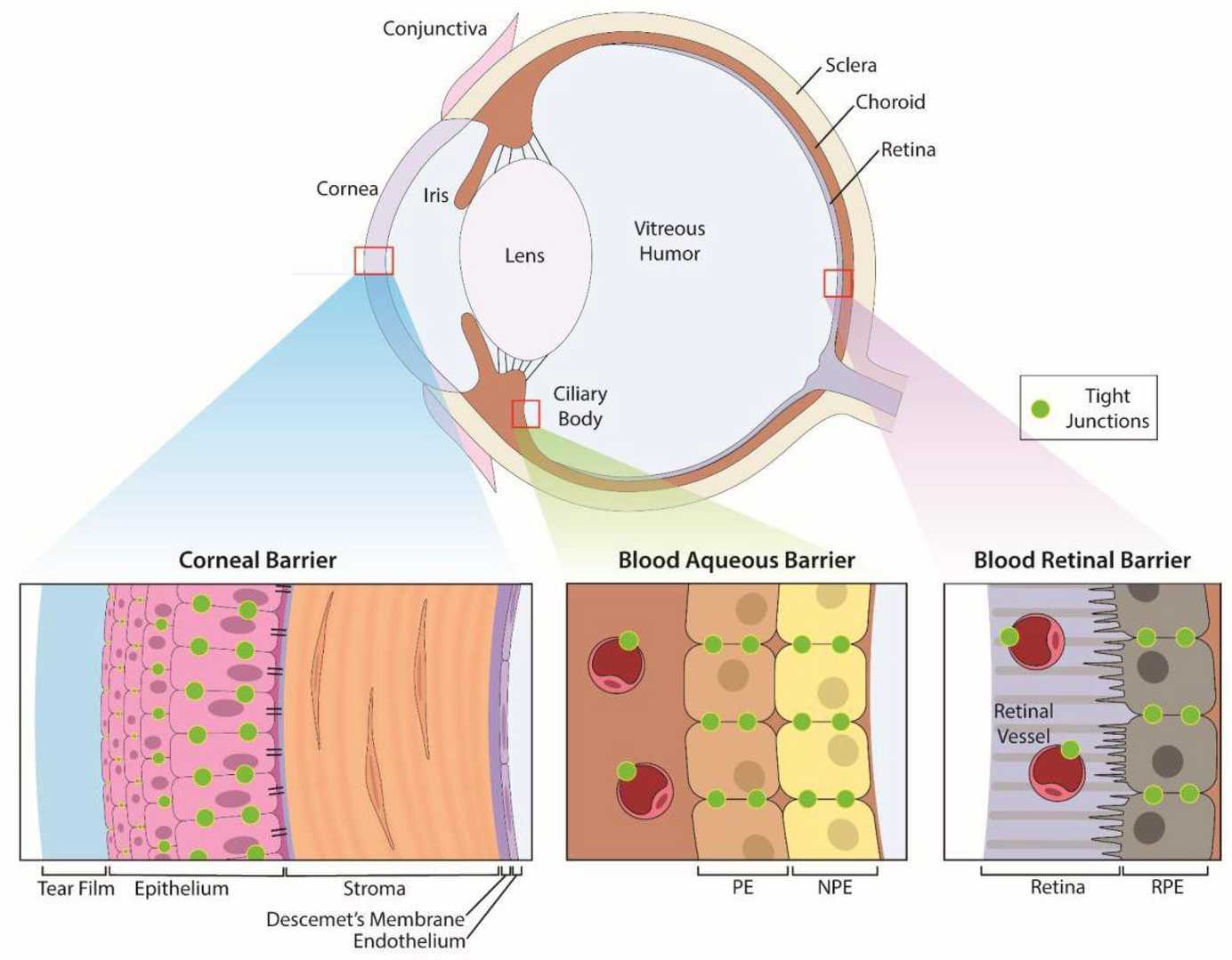 Fig. 1. Anatomic structures of the eye and its specific ocular barriers (Cosert KM, Kim S, et al., 2022).
Fig. 1. Anatomic structures of the eye and its specific ocular barriers (Cosert KM, Kim S, et al., 2022).
Our ocular toxicity evaluation services are designed to support pharmaceutical, biotech, and chemical industry clients in identifying and mitigating ocular risks early in the development process. We have a wide selection of in vitro and in vivo models that are appropriate for regulatory evaluation as well as customized research projects.
Applications
Our ocular toxicity testing services support a wide range of development needs:
- Systemic drugs with off-target ocular risk
- Ophthalmic formulations (e.g., eye drops, ointments, implants)
- Gene and cell therapies targeting retinal or corneal tissues
- Medical devices intended for ocular use
- Cosmetic and personal care products safety assessments
- Agrochemical and industrial compound risk evaluations
Our Ocular Toxicity Services
Ocular irritation and corrosion assays
Our validated suite of assays can be used to evaluate the potential for eye irritation/corrosion of chemicals, drug candidates, or formulations to support regulatory classification and labeling:
OECD TG 405
Acute Eye Irritation/Corrosion Test in vivo (Draize test) with rabbits. This is the gold standard OECD test to evaluate irreversible ocular damage.
OECD TG 437
BCOP (Bovine Corneal Opacity and Permeability) Assay for identification of serious eye damage in isolated bovine corneas.
OECD TG 438
ICE (Isolated Chicken Eye) Test for identification of serious eye damage.
OECD TG 491
Reconstructed Human Cornea-like Epithelium (RhCE) Test Method for identification of chemicals that are not required for classification for eye irritation.
Histopathological assessment of ocular tissues
Our board-certified pathologists perform comprehensive histopathological evaluation of ocular tissues to identify microstructural changes induced by the test article. This analysis is routinely offered as part of systemic toxicity studies or standalone ocular toxicity studies.
- Processing and sectioning of ocular tissues such as cornea, lens, retina, iris, optic nerve, etc.
- Application of H&E staining, special stains (e.g., PAS), and IHC markers if required
- Assessment of inflammation, degeneration, neovascularization, and other lesions
- Correlation with functional endpoints and clinical observations
Slit-lamp biomicroscopy & indirect ophthalmoscopy
These non-invasive ophthalmic techniques are utilized to evaluate clinical signs of ocular toxicity in live animals throughout preclinical studies.
- Slit-lamp biomicroscopy allows high-resolution imaging of the anterior segment (cornea, conjunctiva, iris, lens)
- Indirect ophthalmoscopy enables detailed visualization of the retina and optic nerve
- Performed at multiple timepoints to monitor progression or recovery
- Applicable to rabbit, rodent, dog, and non-human primate models
Electroretinography (ERG) for retinal function
ERG is a sophisticated, quantitative technique that measures retinal electrophysiological responses to various light stimuli, providing an indication of the functionality of photoreceptors and inner retinal neurons.
- Measures a-wave and b-wave amplitudes and latencies
- Applied in toxicology, gene therapy, and ophthalmic drug development
- Facilitates detection of retinal dysfunction prior to histological changes
- Available for both anesthetized and conscious animal models (e.g., rodents, NHPs)
Available Experimental Models
In Vivo Models
Rabbits
Rodents (rats, mice)
non-human primates (NHPs)…
In Vitro Models
Bovine cornea
Isolated chicken eyes
Reconstructed human corneal epithelium (RhCE)
iPSC-derived retinal pigment epithelium…
Features

Versatile testing platforms
From high-throughput in vitro screens to GLP-compliant in vivo studies.

Expert toxicology team
Over 10 years of experience in ocular pathology and pharmacology.
Quality histopathology
Eye tissues are evaluated by board-certified pathologists with ocular expertise.
Timely studies
Rapid timelines with no sacrifice in data quality.
FAQ
What is ocular toxicity?
Ocular toxicity is the capacity of a substance to cause adverse effects on the eyes. It refers to the potential of a chemical, drug, or other material to cause damage or injury to the various structures and tissues of the eye, including the cornea, conjunctiva, iris, and retina. Ocular toxicity can manifest as a range of symptoms and signs, from mild irritation to severe inflammation or injury, and can occur as a result of acute or chronic exposure to the substance. It is a significant consideration in the safety evaluation and risk assessment of various products, including pharmaceuticals, cosmetics, industrial chemicals, and medical devices.
What are the common symptoms of ocular toxicity?
ymptoms of ocular toxicity can vary depending on the severity and type of exposure. Common symptoms include:
- Redness and swelling of the eye
- Pain or discomfort
- Excessive tearing
- Blurred vision
- Sensitivity to light
- Foreign body sensation
- Corneal opacity or ulceration
- Inflammation of the conjunctiva or iris
In severe cases, ocular toxicity can lead to permanent damage or vision loss.
Is ocular toxicity testing required for non-ophthalmic drugs?
Yes, for systemically administered drugs with a known ocular liability or class effect, regulatory agencies often suggest the inclusion of an ocular assessment in the general toxicity study package.
How do you select the appropriate test model for a compound?
Model selection is determined by the characteristics of the compound, intended route of administration, regulatory requirements, and the overall risk assessment strategy. Our toxicology experts provide consultation to guide model selection.
What is the Reconstructed Human Cornea-like Epithelium (RhCE) test?
The Reconstructed Human Cornea-like Epithelium (RhCE) test is an in vitro assay that uses a three-dimensional tissue construct to mimic the structure and function of the human corneal epithelium. The RhCE test is designed to assess the potential ocular irritation and corrosion of substances, and is known for its high predictive accuracy. It is widely used and accepted for compliance with OECD guidelines.
Reference
- Cosert KM, Kim S, et al. Metallic Engineered Nanomaterials and Ocular Toxicity: A Current Perspective. Pharmaceutics. 2022. 14(5):981.
Explore Other Options