Fructose and Potassium Oxonate-Induced Hyperuricemia Model
Creative Bioarray is renowned for its expertise in establishing innovative animal models that accurately replicate human diseases. Our commitment to excellence is evident in our meticulously developed hyperuricemia model, which is induced through a combination of fructose and potassium oxonate. This model has been carefully designed to not only simulate the pathophysiological conditions of hyperuricemia but also to be adaptable to your specific research needs. Whether you are exploring the intricacies of disease mechanisms, evaluating novel therapeutic interventions, or validating diagnostic tools, our hyperuricemia model offers a robust platform to advance your research endeavors.
The fructose and potassium oxonate-induced hyperuricemia model offers a compelling approach to studying the mechanisms and treatments of hyperuricemia, leveraging the distinct metabolic pathways influenced by fructose and potassium oxonate. Fructose, predominantly metabolized in the liver by ketohexokinase (fructokinase C, KHK-C, or KHK), leads to the rapid generation of fructose-1-phosphate. This swift reaction depletes ATP and lowers phosphorylation levels, prompting an increase in AMP deaminase activity under conditions of reduced intracellular phosphate. Consequently, AMP is converted to IMP, intensifying the production of uric acid.
In tandem, potassium oxonate, a triazabenzene compound, inhibits uricase activity, further elevating uric acid levels. This dual mechanism--fructose metabolism and uricase inhibition--creates a robust model for hyperuricemia that closely mimics the human condition.
Our Fructose and Potassium Oxonate-Induced Hyperuricemia Model
- Available Animal
Rat
- Modeling Method
Animals are administered a daily regimen of fructose dissolved in drinking water, supplemented with intragastric potassium oxonate, over a period of 10 weeks to induce hyperuricemia.
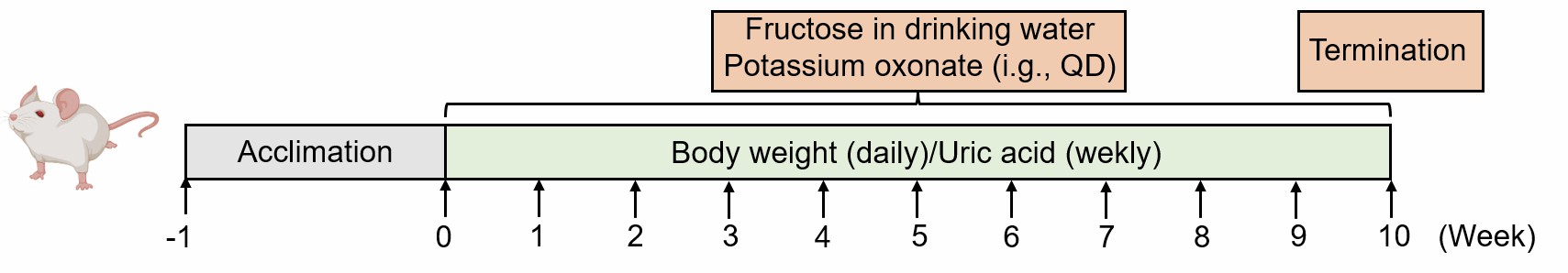 Fig. 1 Modeling method of fructose and potassium oxonate-induced hyperuricemia model.
Fig. 1 Modeling method of fructose and potassium oxonate-induced hyperuricemia model.
- Endpoints
- Serum biomarkers: uric acid (UA), creatinine, BUN, etc
- Kidney observation
- Histology analysis of kidney: H&E staining, Masson staining
- qPCR or Western blot
- Body weight
- Other customized endpoints: available upon request
Quotation and Ordering
Creative Bioarray is willing to share our state-of-the-art platforms and sufficient expertise with our clients to boost their drug development. If you are interested in our services, please do not hesitate to contact us at any time or submit an inquiry to us directly.
Reference
- Xu, Z., et al. Comparison of 3 hyperuricemia mouse models and evaluation of food-derived anti-hyperuricemia compound with spontaneous hyperuricemia mouse model. Biochemical and Biophysical Research Communications, 2022, 630: 41-49.
For research use only. Not for any other purpose.
Disease Models
- Oncology Models
-
Inflammation & Autoimmune Disease Models
- Rheumatoid Arthritis Models
- Glomerulonephritis Models
- Multiple Sclerosis (MS) Models
- Ocular Inflammation Models
- Sjögren's Syndrome Model
- LPS-induced Acute Lung Injury Model
- Peritonitis Models
- Passive Cutaneous Anaphylaxis Model
- Delayed-Type Hypersensitivity (DTH) Models
- Inflammatory Bowel Disease Models
- Systemic Lupus Erythematosus Animal Models
- Oral Mucositis Model
- Asthma Model
- Sepsis Model
- Psoriasis Model
- Atopic Dermatitis (AD) Model
- Scleroderma Model
- Gouty Arthritis Model
- Carrageenan-Induced Air Pouch Synovitis Model
- Carrageenan-Induced Paw Edema Model
- Experimental Autoimmune Myasthenia Gravis (EAMG) Model
- Graft-versus-host Disease (GvHD) Models
-
Cardiovascular Disease Models
- Surgical Models
- Animal Models of Hypertension
- Venous Thrombosis Model
- Atherosclerosis model
- Cardiac Arrhythmia Model
- Hyperlipoidemia Model
- Doxorubicin-induced Heart Failure Model
- Isoproterenol-induced Heart Failure Model
- Arterial Thrombosis Model
- Pulmonary Arterial Hypertension (PAH) Models
- Heart Failure with Preserved Ejection Fraction (HFpEF) Model
- Cardio-Renal-Metabolic (CKM) Syndrome Model
-
Neurological Disease Models
- Alzheimer's Disease Modeling and Assays
- Seizure Models
- Parkinson's Disease Models
- Ischemic Stroke Models
- Acute Spinal Cord Injury (ASCI) Model
- Traumatic Brain Injury (TBI) Model
- Hypoxic-Ischemic Encephalopathy (HIE) Model
- Tourette Syndrome (TS) Model
- Amyotrophic Lateral Sclerosis (ALS) Model
- Huntington's Disease (HD) Model
- Intracerebral hemorrhage (ICH) Models
- Schizophrenia Model
- Depression Models
- Pain Models
-
Metabolic Disease Models
- Type 1 Diabetes Mellitus Model
- Type 2 Diabetes Mellitus Model
- Animal Model of Hyperuricemia
-
Nonalcoholic Fatty Liver Disease Model
- High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- Methionine and Choline Deficient (MCD) Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- Gubra-Amylin NASH (GAN) Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- Streptozotocin (STZ) Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- High Fat Diet-Induced Obesity Model
- Diabetic Foot Ulcer (DFU) Model
- Cardio-Renal-Metabolic (CKM) Syndrome Model
- Liver Disease Models
- Rare Disease Models
- Respiratory Disease Models
- Digestive Disease Models
-
Urology Disease Models
- Cisplatin-induced Nephrotoxicity Model
- Unilateral Ureteral Obstruction Model
- 5/6 Nephrectomy Model
- Renal Ischemia-Reperfusion Injury (RIRI) Model
- Diabetic Nephropathy (DN) Models
- Passive Heymann Nephritis (PHN) Model
- Adenine-Induced Chronic Kidney Disease (CKD) Model
- Kidney Stone Model
- Doxorubicin-Induced Nephropathy Model
- Orthotopic Kidney Transplantation Model
- Benign Prostatic Hyperplasia (BPH) Model
- Peritoneal Fibrosis Model
- Cardio-Renal-Metabolic (CKM) Syndrome Model
- Orthopedic Disease Models
- Ocular Disease Models
- Skin Disease Models
- Infectious Disease Models
- Otology Disease Models