Human Hybridoma Cells
- Background
- Applications
- Scientific Data
- FAQ
Human hybrid cells, formed by fusing human B lymphocytes with mouse myeloma cells, have emerged as powerful tools that are reshaping the landscape of biomedical applications. They are a key component of hybridoma technology, which is a method for producing large numbers of identical antibodies, also known as monoclonal antibodies. The hybridoma cells possess a captivating blend of characteristics that set them apart from their parental cell lines. In addition, human hybridoma cells have the advantage of being more compatible with the human immune system, reducing the risk of adverse reactions when the antibodies are used in therapeutic applications.
Structure and Properties of Human Hybridoma Cells
- Cellular composition and morphology. Human hybridoma cells possess a large, irregularly shaped appearance, with a high nuclear-to-cytoplasmic ratio that reflects their heightened transcriptional activity. The centrally located nucleus, often accompanied by multiple nucleoli, is a testament to the cell's dedication to producing high levels of antibodies – a hallmark of their unique genetic makeup. The cytoplasm of human hybridoma cells is abundant and teeming with secretory vesicles and organelles, further emphasizing their role as prolific antibody factories. These cells also have a propensity to grow in clusters or clumps, a characteristic that sets them apart from their parental cell lines and serves as a reliable identifier during cell culture and isolation processes.
- Genetic and molecular characteristics. At the molecular level, human hybridoma cells express a unique combination of human and mouse cell surface markers, including B cell-specific markers like CD19 and CD20, as well as myeloma-specific markers such as CD138. This mosaic of cellular signatures is a testament to the successful fusion of these two distinct cell types, creating a hybrid that retains the valuable attributes of both parent cells. The antibodies produced by human hybridoma cells are predominantly of the IgG or IgM class, reflecting their B cell origin. Importantly, the versatility of these cells allows for the generation of antibodies with a wide range of antigen specificities, depending on the original B cell that was fused.
Monoclonal Antibody Production
The ability of human hybridoma cells to produce high-affinity, human-derived monoclonal antibodies has revolutionized the development of targeted therapeutics and diagnostic agents. These antibodies can be utilized in the treatment of a wide range of diseases, from cancer to autoimmune disorders.
Immunological Studies
The unique genetic and molecular characteristics of human hybridoma cells make them invaluable tools for investigating the intricate workings of the human immune system. Human hybridoma cells provide a valuable in vitro system for exploring the intricacies of the human immune system. Researchers can utilize these cells to investigate B cell development, antigen recognition, and antibody secretion mechanisms. This knowledge can inform the development of novel immunotherapies, vaccines, and diagnostic tools.
Vaccine Development
Human hybridoma cells can be employed to generate human-derived antibodies against specific pathogens, such as viruses, bacteria, and toxins. These antibodies can be used as passive immunotherapies or as part of vaccine formulations, leveraging the human-specific immune responses they elicit. The ability to rapidly produce large quantities of these antibodies makes human hybridoma cells invaluable in the rapid response to emerging infectious diseases.
Effects of IPSF Activity of the Histone on Human-Human Hybridoma HB4C5 Cells
Regarding the mode of action of histone as an immunoglobulin production stimulating factor (IPSF), a question is whether the conformation of the histone takes a part in its IPSF activity or not. Histone was digested by various concentrations of trypsin or chymotrypsin, and the IPSF activity of these digested histones (Fig. 1). Histone was treated with 10-2-103 USP units/ml of trypsin for 10 min, and the digestion was stopped by addition of 1300 mg/ml of trypsin inhibitor to the reaction mixture. The trypsin-treated histone was analyzed by SDS-PAGE (Fig. 1A). As shown in Fig. 1B, the IPSF activity was lost as a consequence of the fragmentation of the histone.
Next, histone was digested by various concentrations of chymotrypsin, and the IPSF activity of these digested histones (Fig. 2). Histone was treated with 8×10-5-8 units/ml of chymotrypsin, and the digestion was stopped by addition of 10 μg/ml of chymotrypsin inhibitor (TPCK) to the reaction mixture. The fragments derived from histone were analyzed by SDS-PAGE (Fig. 2A). The IPSF activity was lost as a consequence of the fragmentation of the histone (Fig. 2B). These results suggest that the native structure of histone is required for the IPSF activity.
![[A] Histone was directly labeled by FITC. FITC-conjugated histone was digested by various concentrations of trypsin. Following the digestive reaction at 37°C, histone fragments were assayed by SDS-PAGE. The bands of fragments derived from histone were seen by UV light. [B] The IPSF activity of histone digested by trypsin for various periods was assayed. The bars indicate the IgM production of each reaction mixture.](/upload/image/3-human-hybridoma-cells-1.jpg) Fig.
1 Effects of trypsin digestion on IPSF activity of histone. (Okamoto T, et al, 2002)
Fig.
1 Effects of trypsin digestion on IPSF activity of histone. (Okamoto T, et al, 2002)
![[A] Histone was directly labeled by FITC. FITC-conjugated histone was digested by various concentrations of chymotrypsin. Following a digestive reaction at 37°C, histone fragments were assayed by SDS-PAGE. The bands of fragments derived from histone were seen by UV light. [B] The IPSF activities of histone digested by chymotrypsin for various periods were assayed. The bars indicate the IgM production of each reaction mixture.](/upload/image/3-human-hybridoma-cells-2.jpg) Fig.
2 Effects of chymotrypsin digestion on IPSF activity of histone. (Okamoto T, et al, 2002)
Fig.
2 Effects of chymotrypsin digestion on IPSF activity of histone. (Okamoto T, et al, 2002)
Human Hybridoma Preparation Using Human Myeloma Cell Line Karpas707H
Human-human hybridoma was established by fusing B cells prepared from the spleen of an immunized CB-NOG mouse with a human myeloma cell line. As for the human myeloma cell line, Karpas707H was used. The specificity of antibodies secreted in the culture supernatant was analyzed by ELISA, the results of which are shown in Table 1. Only four Her-2-specific hybridoma lines were obtained from the mice immunized with exHer-2, while 32 hybridoma lines were obtained for DNP-KLH-specific B cells. As CB-NOG mice produce mainly IgM isotypes for antigen-specific antibodies, the spleen cells of the mice immunized with Her-2 peptide and SV22 were transformed with EBV before the fusion with Karpas707H. As a result, specific antibody-producing hybridoma lines were obtained in both experiments, with or without EBV treatment. Consequently, a total of 20 hybridoma lines producing peptide-specific antibodies were obtained from the mice (P/S-1, S, and P/S-2). Moreover, 28 peptide-specific IgM-producing hybridoma lines were obtained in P/S-3 mice. One of these clones was expanded and anti-peptide IgM was collected from the supernatants. FACS analysis showed that the antibody specifically recognized surface Her-2 expressed on A20, though the intensity was not so high as that of Herceptin (Fig. 3).
Table 1. Summary of hybridoma production by CB-NOG B cells and Karpas707H.
| Ag | Mouse | EBV | Cell (×105) | Colony (n) | Total antibody (colony, n) | Specific antibody (colony, n) | ||
| lgM | lgG | lgM | lgG | |||||
| H | 4 | - | 921 | 142 | 21 | 15 | 4 | ND |
| P/S-1 | 1 | + | 366 | 31 | ND | ND | 8 | ND |
| P/S-2 | 1 | + | 255 | 14 | ND | ND | 2 | ND |
| P/S-3 | 1 | + | 641 | 54 | ND | ND | 28 | ND |
| S | 1 | + | 358 | 48 | ND | ND | 10 | ND |
| DNP-KLH | 4 | - | 351 | 58 | 32 | 0 | 32 | 0 |
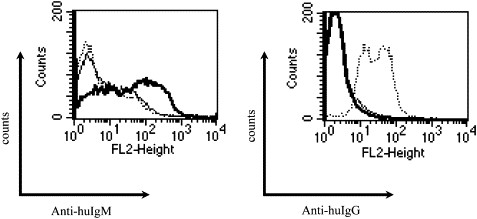 Fig.
3 Recognition of surface Her-2 by human anti-Her-2 immunoglobulin M (IgM). Anti-Her-2 IgM was prepared from
human hybridoma and purified. (Kametani Y, et al, 2006)
Fig.
3 Recognition of surface Her-2 by human anti-Her-2 immunoglobulin M (IgM). Anti-Her-2 IgM was prepared from
human hybridoma and purified. (Kametani Y, et al, 2006)
Human hybridoma cells are created by fusing antigen-specific human B-cells with human or mouse myeloma cells. This fusion allows the hybridoma cells to produce specific antibodies while also having the ability to replicate indefinitely.
The main advantage of using human hybridoma cells is that they produce human antibodies, which are more compatible with the human immune system and less likely to cause adverse reactions when used in therapeutic applications.
Human hybridoma cells are used in research and therapeutic applications because they can produce large quantities of specific monoclonal antibodies. This makes them highly valuable in the treatment of various diseases, including cancers, autoimmune diseases, and infectious diseases.
Filters Clear all filters
Species
- African clawed frog (1)
- American mink (1)
- Asian tiger mosquito (1)
- Atlantic salmon (1)
- Bluegill (2)
- Bluestriped grunt (1)
- Bovine (7)
- Brazilian free-tailed bat (1)
- Brown bullhead (2)
- Cabbage looper (1)
- Cabbage moth (6)
- Cat (4)
- Central mudminnow (1)
- Chicken (3)
- Chinese hamster (5)
- Chinook salmon (2)
- Chum salmon (1)
- Coho salmon (1)
- Common carp (2)
- Cotton-top tamarin (1)
- Dog (2)
- Fall armyworm (3)
- Fathead minnow (2)
- Fruit fly (1)
- Gilthead sea bream (2)
- Golden hamster (7)
- Goldfish (6)
- Gray dwarf hamster (1)
- Green monkey (2)
- Gypsy moth (1)
- Horse (1)
- Human (998)
- Japanese eel (1)
- Japanese rice fish (7)
- Koi carp (1)
- Mouse (315)
- Mouse x Gray dwarf hamster (1)
- Mouse x Rat (20)
- Northern pike (1)
- Pig (3)
- Rabbit (2)
- Rainbow trout (3)
- Rat (115)
- Rhesus macaque (1)
- Salt marsh moth (1)
- Sheep (2)
- Snakehead murrel (2)
- Sockeye salmon (1)
- Vervet monkey (2)
- Zebrafish (2)
Source
- Abdomen (1)
- Abdomen Metastasis (2)
- Adipose (2)
- Adrenal Gland (8)
- Adrenal Gland Metastasis (2)
- Aorta (4)
- Artery (1)
- Ascites (28)
- Ascites Metastasis (37)
- Bile Duct (3)
- Bladder (25)
- Bladder Metastasis (1)
- Blastocyst (1)
- Blastula (1)
- Blood (127)
- Bone (27)
- Bone Marrow (57)
- Bone Marrow Metastasis (18)
- Bone Metastasis (6)
- Brain (55)
- Brain Metastasis (8)
- Breast (30)
- Bronchus (1)
- Caudal Peduncle (1)
- Caudal Trunk (2)
- Cecum (3)
- Cerebrospinal Fluid (1)
- Cerebrospinal Fluid Metastasis (1)
- Cervix (32)
- Colon (90)
- Connective Tissue (7)
- Cornea (3)
- Cutaneous Metastasis (1)
- Dermis (2)
- Duodenum (1)
- Embryo (29)
- Endometrium (17)
- Esophagus (44)
- Eye (12)
- Eye Socket (5)
- Fetus (3)
- Fin (9)
- Foreskin (4)
- Gallbladder (1)
- Gingiva (2)
- Globe (2)
- Glomerulus (2)
- Groin (1)
- Head Kidney (2)
- Heart (4)
- Hemolymph (1)
- Hypodermis Metastasis (5)
- Ileum (1)
- Intestine (94)
- Jejunum (1)
- kidney (1)
- Kidney (27)
- Liver (35)
- Liver Metastasis (17)
- Lung (58)
- Lung Metastasis (8)
- Lymph Node (7)
- Lymph Node Metastasis (59)
- Muscle (7)
- Muscle Metastasis (2)
- Nose (2)
- Omentum Metastasis (2)
- Oral Cavity (10)
- Ovary (21)
- Ovary Metastasis (2)
- Pancreas (19)
- Pelvic Wall Metastasis (1)
- Pelvis (1)
- Perianal Space Metastasis (1)
- Pericardial Effusion (1)
- Pericardial Effusion Metastasis (2)
- Perineus (1)
- Peripheral Blood (126)
- Peripheral Nervous System (21)
- Peritoneal Effusion (2)
- Peritoneum (1)
- Peritoneum Metastasis (1)
- Pharynx (3)
- Pituitary Gland (7)
- Pleural Effusion (54)
- Pleural Effusion Metastasis (46)
- Prostate (7)
- Rectum (15)
- Renal Pelvis (1)
- Retroperitoneal Space (2)
- Salivary Gland (2)
- Skeletal Muscle (5)
- Skin (32)
- Skin Metastasis (3)
- Small Intestine (4)
- Small Intestine Metastasis (1)
- Smooth Muscle (2)
- Soft Tissue (1)
- Soft Tissue Metastasis (1)
- Spinal Cord (2)
- Stomach (4)
- Testis (15)
- Thoracic Cavity Metastasis (6)
- Thymus (5)
- Thyroid Gland (16)
- Thyroid Gland Metastasis (1)
- Tongue (5)
- Trachea (1)
- Umbilical Cord (1)
- Umbilical Cord Blood (1)
- Urachus (1)
- Ureter (1)
- Uterus (54)
- Uvea (2)
- Vagina (2)
- Vulva (1)
Disease
- Acute Biphenotypic Leukemia (1)
- Acute Erythroid Leukemia (4)
- Acute Megakaryoblastic Leukemia (4)
- Acute Monocytic Leukemia (9)
- Acute Myeloid Leukemia (25)
- Acute Promyelocytic Leukemia (2)
- Adrenal Gland Neuroblastoma (11)
- Adult B Acute Lymphoblastic leukemia (1)
- Adult B Acute Lymphoblastic Leukemia (6)
- Adult T Acute Lymphoblastic Leukemia (6)
- Adult T Lymphoblastic Lymphoma (2)
- Adult T-Cell Leukemia/Lymphoma (1)
- Alveolar Rhabdomyosarcoma (4)
- Alveolar Ridge Squamous Cell Carcinoma (1)
- Amelanotic Melanoma (3)
- Ampulla of Vater Adenocarcinoma (1)
- Ampulla of Vater Adenosquamous Carcinoma (3)
- Anaplastic Astrocytoma (3)
- Anaplastic Large Cell Lymphoma (7)
- Askin Tumor (1)
- Astrocytoma (5)
- B Acute Lymphoblastic Leukemia (2)
- B-Cell Non-Hodgkin Lymphoma (5)
- Bare Lymphocyte Syndrome Type 2 (1)
- Barrett Adenocarcinoma (2)
- Benign Prostatic Hyperplasia (1)
- Bladder Carcinoma (14)
- Bladder Squamous Cell Carcinoma (1)
- Bovine Leukemia (2)
- Breast Adenocarcinoma (4)
- Breast Carcinoma (9)
- Breast Ductal Carcinoma (2)
- Burkitt Lymphoma (17)
- Canavan Disease (1)
- Canine Histiocytic Sarcoma (1)
- Cecum Adenocarcinoma (3)
- Central Nervous System Lymphoma (2)
- Cervical Adenocarcinoma (2)
- Cervical Adenosquamous Carcinoma (2)
- Cervical Small Cell Carcinoma (1)
- Cervical Squamous Cell Carcinoma (2)
- Chicken Bursal Lymphoma (2)
- Childhood B Acute Lymphoblastic Leukemia (13)
- Childhood T Acute Lymphoblastic Leukemia (16)
- Childhood T Lymphoblastic Lymphoma (1)
- Cholangiocarcinoma (2)
- Chronic Eosinophilic Leukemia (1)
- Chronic Lymphocytic Leukemia (2)
- Chronic Myeloid Leukemia (23)
- Clear Cell Renal Cell Carcinoma (2)
- Colon Adenocarcinoma (55)
- Colon Carcinoma (34)
- Colorectal Adenocarcinoma (1)
- Colorectal Carcinoma (1)
- Congenital Pure Red Cell Aplasia (1)
- Cutaneous Melanoma (10)
- Dedifferentiated Chondrosarcoma (1)
- Desmoplastic Melanoma (1)
- Diffuse Large B-Cell Lymphoma (28)
- Down Syndrome (2)
- EBV-Related Burkitt Lymphoma (12)
- Embryonal Carcinoma (3)
- Embryonal Rhabdomyosarcoma (3)
- Endometrial Adenocarcinoma (13)
- Endometrial Adenosquamous Carcinoma (2)
- Endometrial Carcinoma (2)
- Endometrioid Stromal Sarcoma (1)
- Epithelioid Hemangioendothelioma (1)
- Epithelioid Sarcoma (3)
- Esophageal Adenocarcinoma (6)
- Esophageal Squamous Cell Carcinoma (41)
- Essential Thrombocythemia (1)
- Ewing Sarcoma (2)
- Extraskeletal Myxoid Chondrosarcoma (1)
- Fanconi Anemia (1)
- Fibrosarcoma (1)
- Follicular Lymphoma (2)
- Gallbladder Carcinoma (2)
- Gallbladder Undifferentiated Carcinoma (2)
- Gastric Adenocarcinoma (6)
- Gastric Adenosquamous Carcinoma (1)
- Gastric Carcinoma (5)
- Gastric Choriocarcinoma (1)
- Gastric Fundus Carcinoma (1)
- Gastric Signet Ring Cell Adenocarcinoma (1)
- Gastric Small Cell Carcinoma (2)
- Gastric Tubular Adenocarcinoma (5)
- Gastroesophageal Junction Adenocarcinoma (1)
- Gestational Choriocarcinoma (1)
- Gingival Squamous Cell Carcinoma (2)
- Glioblastoma (18)
- Gliosarcoma (1)
- Goldfish Erythrophoroma (4)
- Hairy Cell Leukemia (1)
- Hamster Kidney Tumor (1)
- Hamster Pancreatic Ductal Adenocarcinoma (1)
- Hamster Uterine Leiomyosarcoma (1)
- Hepatoblastoma (2)
- Hepatocellular Carcinoma (6)
- Hepatosplenic T-Cell Lymphoma (2)
- Hereditary Thyroid Gland Medullary Carcinoma (1)
- High Grade B-Cell Lymphoma (1)
- High Grade Ovarian Serous Adenocarcinoma (8)
- Hodgkin Lymphoma (9)
- Hypopharyngeal Squamous Cell Carcinoma (2)
- Infectious Mononucleosis (1)
- Intrahepatic Cholangiocarcinoma (6)
- Invasive Breast Carcinoma of No Special Type (12)
- Invasive Breast Lobular Carcinoma (1)
- Kidney Neoplasm (1)
- Kidney Rhabdoid Tumor (1)
- Krukenberg Tumor (1)
- Liposarcoma (1)
- Lung Adenocarcinoma (17)
- Lung Giant Cell Carcinoma (8)
- Lung Large Cell Carcinoma (9)
- Lung Mucoepidermoid Carcinoma (1)
- Lung Non-Small Cell Carcinoma (2)
- Lung Small Cell Carcinoma (25)
- Lung Squamous Cell Carcinoma (9)
- Lymphoblastic Lymphoma (1)
- Malignant Peripheral Nerve Sheath Tumor (1)
- Mantle Cell Lymphoma (5)
- Mature Gastric Teratoma (1)
- Maxillary Sinus Squamous Cell Carcinoma (1)
- Medaka Hepatoma (2)
- Medulloblastoma (3)
- Melanoma (24)
- Meningioma (2)
- Minimally Invasive Lung Adenocarcinoma (1)
- Monophasic Synovial Sarcoma (1)
- Mouse Bladder Transitional Cell Carcinoma (1)
- Mouse Chondrosarcoma (1)
- Mouse Colon Adenocarcinoma (3)
- Mouse Ependymoma (2)
- Mouse Erythroid Leukemia (13)
- Mouse Fibrosarcoma (5)
- Mouse Glioblastoma (1)
- Mouse Hemangioendothelioma (1)
- Mouse Hepatocellular Carcinoma (1)
- Mouse Insulinoma (3)
- Mouse Intestinal Tract Neuroendocrine Adenoma (1)
- Mouse Islet Cell Adenoma (1)
- Mouse Kidney Carcinoma (1)
- Mouse Leukemia (10)
- Mouse Leydig Cell Tumor (1)
- Mouse Lymphoma (8)
- Mouse Mammary Gland Malignant Neoplasm (23)
- Mouse Melanoma (9)
- Mouse Multiple Myeloma (5)
- Mouse Myeloid Leukemia (3)
- Mouse Neoplasm (1)
- Mouse Neuroblastoma (21)
- Mouse Oral Cavity Squamous Cell Carcinoma (1)
- Mouse Osteosarcoma (3)
- Mouse Pituitary Gland Neoplasm (1)
- Mouse Plasmacytoma (1)
- Mouse Precursor T Cell Lymphoblastic Lymphoma/Leukemia (2)
- Mouse Pulmonary Adenoma (1)
- Mouse Pulmonary Malignant Tumor (3)
- Mouse Pulmonary Squamous Cell Carcinoma (1)
- Mouse Rectum Carcinoma (2)
- Mouse Reticulum Cell Sarcoma (2)
- Mouse Sarcoma (1)
- Mouse Teratocarcinoma (8)
- Mouse Thymic Lymphoma (3)
- Mycosis Fungoides (1)
- Myelodysplastic Syndrome (1)
- Myxofibrosarcoma (1)
- Natural Killer Cell Lymphoblastic Leukemia/Lymphoma (2)
- Neuroblastoma (26)
- Oral Cavity Squamous Cell Carcinoma (15)
- Osteoid Osteoma (1)
- Osteosarcoma (15)
- Ovarian Carcinoma (1)
- Ovarian Clear Cell Adenocarcinoma (1)
- Ovarian Endometrioid Adenocarcinoma (4)
- Ovarian Granulosa Cell Tumor (1)
- Ovarian Mucinous Adenocarcinoma (2)
- Ovarian Serous Adenocarcinoma (2)
- Ovarian Serous Cystadenocarcinoma (2)
- Ovarian Small Cell Carcinoma (1)
- Pancreatic Adenocarcinoma (13)
- Pancreatic Carcinoma (5)
- Pancreatic Ductal Adenocarcinoma (12)
- Papillomavirus-Independent Cervical Squamous Cell Carcinoma (1)
- Papillomavirus-Related Cervical Adenocarcinoma (7)
- Papillomavirus-Related Cervical Squamous Cell Carcinoma (4)
- Papillomavirus-Related Endocervical Adenocarcinoma (16)
- Paroxysmal Nocturnal Hemoglobinuria (3)
- Pharyngeal Squamous Cell Carcinoma (1)
- Plasma Cell Myeloma (15)
- Pleural Epithelioid Mesothelioma (5)
- Pleural Sarcomatoid Mesothelioma (2)
- Poorly Differentiated Thyroid Gland Carcinoma (1)
- Primary Cutaneous T-Cell Non-Hodgkin Lymphoma (1)
- Primary Effusion Lymphoma (7)
- Primitive Neuroectodermal Tumor (1)
- Prostate carcinoma (1)
- Prostate Carcinoma (9)
- Rat C-Cell Carcinoma (1)
- Rat Cholangiocarcinoma (1)
- Rat Colon Adenocarcinoma (5)
- Rat Digestive System Neoplasm (1)
- Rat Fibrosarcoma (1)
- Rat Hepatocellular Carcinoma (20)
- Rat Histiocytic Sarcoma (1)
- Rat Insulinoma (2)
- Rat Leukemia (1)
- Rat Leydig Cell Adenoma (1)
- Rat Lung Carcinoma (1)
- Rat Malignant Glioma (4)
- Rat Malignant Meningioma (1)
- Rat Malignant Oligodendroglioma (2)
- Rat Malignant Thymoma (3)
- Rat Mammary Gland Adenocarcinoma (10)
- Rat Neuroblastoma (3)
- Rat Osteosarcoma (2)
- Rat Pituitary Gland Neoplasm (6)
- Rat Prostate Adenocarcinoma (3)
- Rat Rhabdomyosarcoma (1)
- Rat Sarcoma (2)
- Rat Squamous Cell Carcinoma (1)
- Rat Urinary Bladder Transitional Cell Carcinoma (2)
- Rat Urinary System Neoplasm (6)
- Rectal Adenocarcinoma (13)
- Rectosigmoid Adenocarcinoma (1)
- Recurrent Bladder Carcinoma (1)
- Renal Cell Carcinoma (7)
- Renal Pelvis Urothelial Carcinoma (1)
- Retinoblastoma (11)
- Sacral Chordoma (1)
- Sacrococcygeal Teratoma (1)
- Salivary Gland Squamous Cell Carcinoma (1)
- Sezary Syndrome (1)
- Shwachman-Diamond Syndrome (1)
- Skin Squamous Cell Carcinoma (2)
- Splenic Marginal Zone Lymphoma (1)
- Testicular Embryonal Carcinoma (8)
- Testicular Teratoma (2)
- Testicular Yolk Sac Tumor (1)
- Thyroid Gland Anaplastic Carcinoma (10)
- Thyroid Gland Follicular Carcinoma (4)
- Thyroid Gland Papillary Carcinoma (3)
- Thyroid Gland Sarcoma (1)
- Thyroid Gland Squamous Cell Carcinoma (2)
- Tongue Adenosquamous Carcinoma (1)
- Tongue Squamous Cell Carcinoma (6)
- Type I Endometrial Adenocarcinoma (1)
- Ureter Urothelial Carcinoma (1)
- Uterine Carcinosarcoma (2)
- Uterine Corpus Leiomyosarcoma (1)
- Uterine Corpus Sarcoma (2)
- Uveal Melanoma (2)
- Vaginal Melanoma (2)
- Vulvar Melanoma (1)
- Vulvar Squamous Cell Carcinoma (1)

