In Vitro Cytotoxicity
- Service Details
- Workflow
- Features
- FAQ
- Explore Other Options
In vitro cytotoxicity testing using cultured cells is an essential tool for the early-stage safety assessment of pharmaceuticals, chemicals, biologics, and medical devices. An in vitro cytotoxicity assay enables the assessment of cell viability together with membrane integrity, cellular metabolic functions, and cell proliferation inhibition following test article exposure. Relative to in vivo assays, in vitro models are a rapid, cost-effective, and ethically sound method for initial toxicity screening, mechanism of action determination and selection of candidate compounds.
By employing cells and cell-based models derived from different tissues and organs, cytotoxicity testing can provide valuable insights into tissue-specific toxicity at an early stage of development. This approach allows high-throughput evaluation of candidate compounds, facilitating the identification of potential liabilities and enabling the prioritization of safer and more promising molecules.
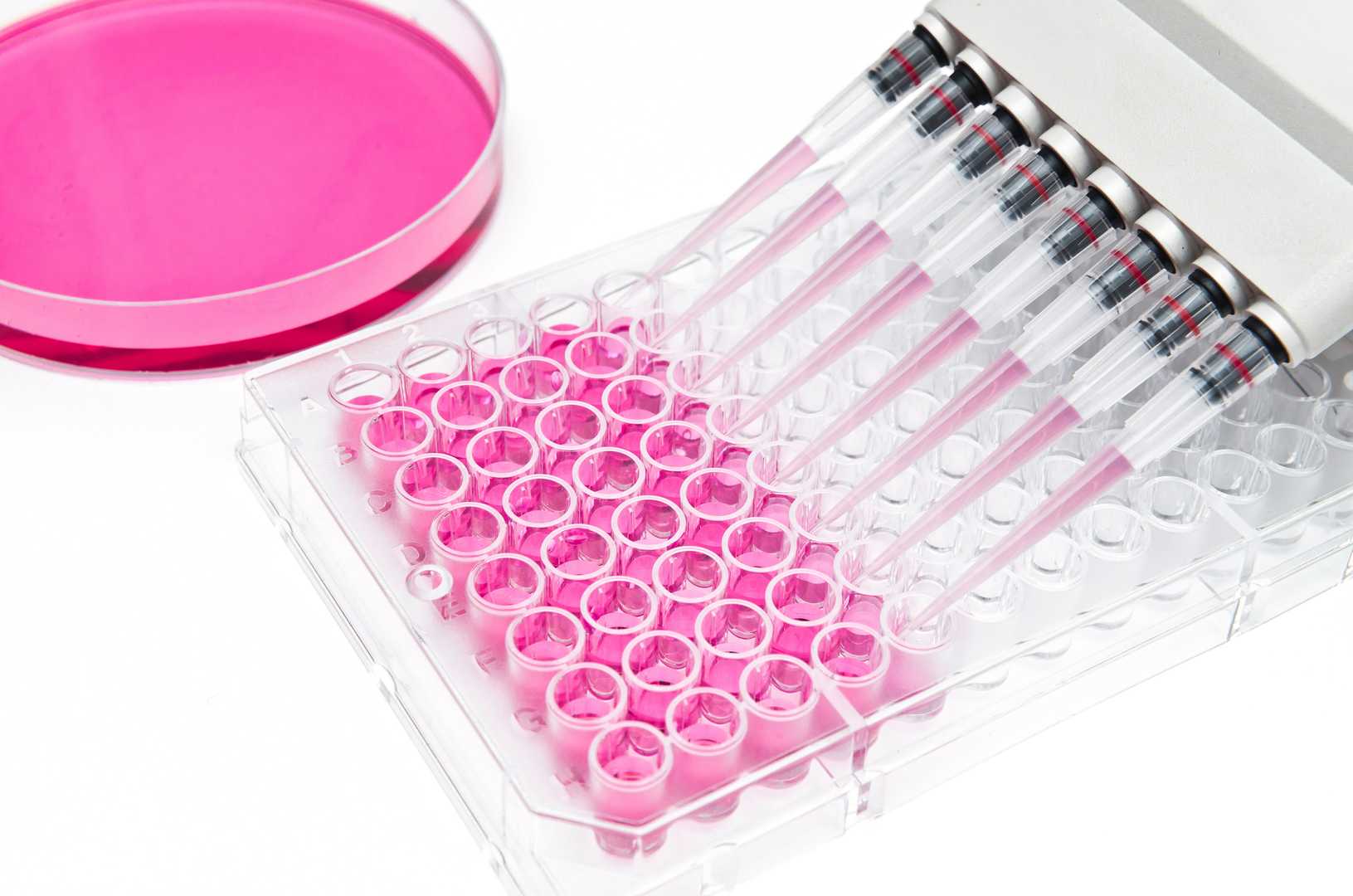
All of our cytotoxicity assays are conducted in accordance with international regulatory guidelines and are designed to help you make informed, data-driven decisions throughout your R&D process.
Our In Vitro Cytotoxicity Service Content:
We offer a comprehensive suite of in vitro cytotoxicity assays, covering multiple toxicological endpoints:
Cell Viability Assays: MTT, WST-1, CCK-8, Resazurin-based (Alamar Blue), Crystal violet staining' etc.
Membrane Integrity Assays: LDH release assay, Trypan Blue exclusion
Apoptosis/Necrosis Detection: Annexin V/PI staining, TUNEL assay, Caspase 3/7 activity
Mitochondrial Function Assays: JC-1 dye for mitochondrial membrane potential, ATP quantification, MitoTracker staining
High-Content Imaging: Multiparametric analysis of cell morphology, membrane damage, and nuclear condensation
Available models
- Standard Cell Lines: Such as HepG2, SH-SY5Y, SK-N-SH, PC-3, HK2, NHBE, HaCaT, L-929, HEK293, A549.
- Primary Cell Lines: Such as primary hepatocytes, renal epithelial cells, cardiomyocytes, hematopoietic cells, PBMCs, bone marrow cells.
- 3D Culture Systems & Organoids: Brain, heart, lung, liver, kidney, and intestinal organoids. Blood vessel, retina. Tumor spheroids for enhanced physiological relevance.
- Microfluidic Cell Culture Systems: Dynamic organ-on-a-chip models for perfusion-based toxicity studies
Workflow
Step 1: Consultation & Project Design
Discuss compound type, exposure route, regulatory goals, and study design.
Step 2: Cell Line Selection & Assay Setup
Choose appropriate cell models and validate assay protocols.
Step 3: Dose-Response Testing
Test multiple concentrations to determine IC₅₀/EC₅₀ or cytotoxicity thresholds.

Step 4: Data Analysis
Statistical analysis, dose-response curve fitting, endpoint comparison.
Step 5: Report Delivery
Detailed study report with raw data, experimental protocol, result interpretation, and representative images.
Features

Flexible & Customizable
Tailored assay design based on compound class, exposure route, and regulatory goals

Experienced Team
Our toxicology scientists are experts in cell-based assay development and data interpretation.
High-Quality Data Output
Validated assays, positive/negative controls and rigorous reproduction to ensure accurate and reliable results.
Fast Turnaround
Optimized workflows and real-time updates to speed up your research timeline.
FAQ
Which samples can be tested in your in vitro cytotoxicity assays?
Small molecules, peptides, biologics, nanoparticles, medical device extracts, and other chemical formulations can all be tested.
What kind of sample should I provide for testing?
Please provide pure samples of your compound (either powder or solution) along with basic physicochemical information, including solubility, to ensure optimal testing conditions.
Do you provide time-dependent toxicity (TDI) testing?
Yes, we can collect data at multiple time points (4 h, 24 h, 48 h, 72 h) to assess acute versus delayed toxicity and calculate AUC-based IC₅₀ values to distinguish the two.
Do you provide high-throughput screening (HTS) capability?
Yes, our fully automated 384-well workstation has the capability for high-throughput testing and can process over 2000 wells per day, making us well suited to assist with compound library screening, QC, and STR studies.
Explore Other Options