When Should You Introduce ADME Tox Testing in Drug Development?
In drug development, the real challenge with ADME tox testing is often not how to run the assays, but when to introduce them. Many programs fail not because ADME tox was ignored, but because it was introduced at the wrong time or applied without a clear purpose.
Some programs have fallen into the trap of introducing ADME tox testing prematurely and found themselves stalled at hit-to-lead with resources spent driving chemists on low quality SAR before the chemistry even stabilized. Others have waited so long that safety liabilities crept in under the radar only to pop up well past Discovery Phase (commonly known as phase killers). These killed programs shared one thing in common - late-stage surprises in ADME/toxicology.
Rather than wax philosophical on the topic of ADME tox testing we thought we'd tackle a more practical question: At what stage of development should your team be introducing ADME tox testing and how much is enough at each stage? We'll approach this topic from the three criteria of project stage, obvious risk flags and decision requirements to come up with some practical guidelines you can actually use rather than a generic "CLICK HERE to Download our ADME-Tox Checklist".
Key Signals: It's Time to Introduce ADME Tox Testing? (When)
You don't always need a full panel on day one. Look for these "risk signals" in your project to know when to pull the trigger.
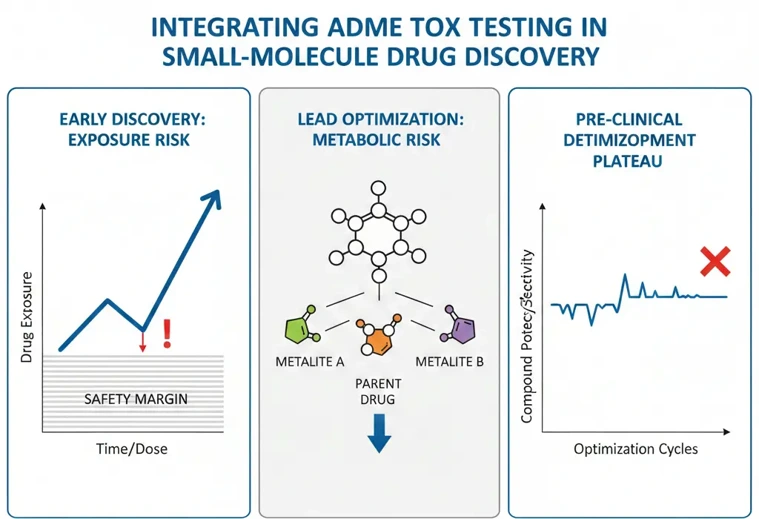
Exposure-Driven Warning Signs
When a compound shows rapid clearance or requires increasingly high doses to maintain exposure, toxicity risk becomes exposure-driven rather than potency-driven. High or repeated dosing increases the likelihood of off-target effects and organ burden, making early safety signals more relevant.
If your PK data suggest narrow exposure margins or compound accumulation, it is usually time to introduce targeted ADME tox testing. In practice, these decisions rely heavily on DMPK studies that clarify whether observed risks are driven by clearance mechanisms, bioavailability limits, or exposure variability.
Metabolism-Related Red Flags
Metabolism is a common source of unexpected toxicity. Warning signs include:
- High metabolic turnover
- Formation of multiple or unusual metabolites
- Large species differences in metabolic pathways
When metabolism becomes unpredictable or difficult to optimize, ADME tox testing helps determine whether safety concerns are driven by the parent compound or by downstream metabolites-an essential component of early safety evaluation, rather than a late-stage toxicology surprise.
Optimization Plateaus
A less obvious but equally important signal is optimization fatigue. If potency continues to improve but exposure or safety margins do not, ADME tox data may be missing from the decision process. At this point, continuing chemistry without safety insight often leads to diminishing returns.
Stage-Based Strategy: A Step-by-Step Approach (How)
1. Lead Optimization (The "Filter" Phase)
At this stage, you don't need GLP-grade studies. You need high-throughput screening (HTS).
What to do: Focus on in silico modeling and simple in vitro assays (like metabolic stability in liver microsomes and basic cytotoxicity).
Goal: Weed out "junk" molecules that won't survive the first pass through the liver.
2. Candidate Selection (The "Predict" Phase)
Now that you have a front-runner, it's time to get serious.
What to do: Move to more complex models. Check for CYP inhibition/induction (drug-drug interactions) and hERG testing for cardiac safety.
Goal: Predict how the drug will behave in a living system and identify any "deal-breakers" before investing in animal models.
3. IND-Enabling Studies (The "Verify" Phase)
This is the formal, regulatory-heavy stage.
What to do: Full GLP-compliant toxicology, definitive PK profiles, and safety pharmacology.
Goal: Prove to the FDA/EMA that your compound is safe enough for human volunteers.
Integrating ADME-Tox with PK and Potency Data
ADME-Tox doesn't live in a vacuum. A molecule with "okay" toxicity but "incredible" potency might still be a winner, whereas a "perfectly safe" molecule that disappears from the blood in ten minutes (poor PK) is useless.
True "Developability" is the intersection of these three metrics. You must weigh potency (does it work?) against pharmacokinetics (does it stay?) and toxicology (is it safe?). If you only track potency, you aren't developing a drug; you're developing a potent poison.
Common Mistakes When Introducing ADME Tox Testing
Despite good intentions, several recurring mistakes reduce the value of ADME tox data:
- Treating ADME tox as a checkbox rather than a decision tool
- Applying late-stage safety criteria too early
- Overreacting to isolated in vitro signals without exposure context
- Ignoring metabolism-driven toxicity risks
- Keeping ADME, PK, and tox data in separate silos
Avoiding these pitfalls requires coordination, timing, and clarity on what each assay is meant to answer.
Frequently Asked Questions (FAQ)
Q: When should drug candidates be introduced to ADME tox during drug development?
A: Not based on a time frame, but rather when certain trends in exposure, metabolism or optimization flag a potential safety risk down the road.
Q: Do all drug candidates undergo ADME tox testing?
A: No, not all drug candidates need to undergo extensive ADME screening and testing. In fact, many early stage programs can get away with very minimal ADME evaluation.
Q: What differentiates ADME tox from standard toxicology studies?
A: Think of ADME tox as performing the safety assessment for drugs in early development. Standard toxicology studies are curated to meet GLP guidelines for regulatory decisions.
Q: Can ADME tox kill projects too early?
A: ABSOLUTELY. Freeing up resources from projects that carry insurmountable safety risks before they take millions of dollars to kill is one of the biggest benefits of ADME tox.
Q: I have access to great in silico tools. Can I just do in silico ADME-Tox instead of in vitro?
A: Partially. While in silico models are great for helping "triage" clear compounds from obvious toxins, biological assays are still needed to rule out adverse effects for regulators.
Q: What is the number one cause of drug failure in ADME-Tox?
A: Metabolism stability (both poor in vitro potency and unexpected drug-drug interactions). Coming in a close second, is hepatotoxicity (liver damage).
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
|---|---|
| Safety Evaluation Services | Creative Bioarray stands as a premier provider of comprehensive safety evaluation studies, adeptly serving a diverse spectrum of industries. Our expertise extends to a broad range of product categories, encompassing pharmaceuticals, cosmetics, and personal care items within the realm of daily chemical products, as well as disinfectants and food products |
| Drug Metabolism and Pharmacokinetics (DMPK) | Creative Bioarray provide comprehensive in vitro and in vivo DMPK solutions to support lead optimization, candidate selection, and regulatory submissions, accelerating the path from discovery to clinical development |