Comparing IHC, ICC, and IF: Which One Fits Your Research?
Immunohistochemistry (IHC), immunocytochemistry (ICC), and immunofluorescence (IF) are three fundamental techniques widely used in life science and medical research. However, many researchers—from beginners to experienced professionals—often struggle to distinguish between them. Are you also wondering:
What are the key differences between IHC, ICC, and IF?
Which technique should I choose for my samples?
Which one is more sensitive? Which one is easier to perform?
In this article, we will provide you a detailed comparison between these 3 techniques including their principles, applications, pros/cons and tips for choosing, to help you select the best method to support your experiments.
What are IHC, ICC, and IF?
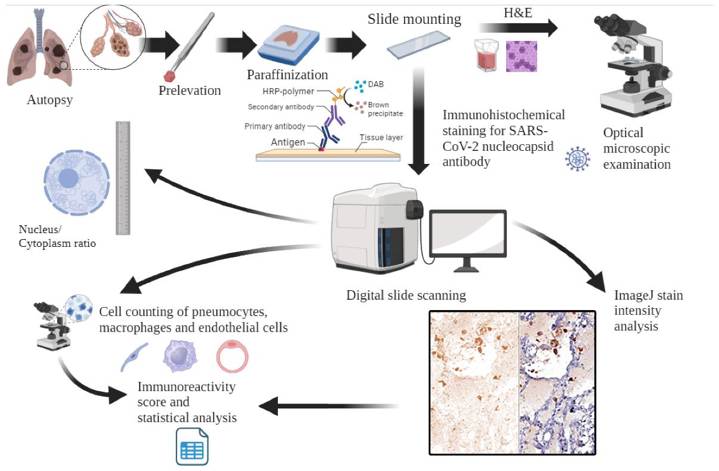
- IHC (Immunohistochemistry) is an immune-detection technique to detect and locate specific antigens (typically proteins) in cells or tissues using the specific binding between antibodies and antigens. It is commonly used in pathology, cancer research, and diagnostics, for example, to study the expression and distribution of specific proteins in tumor tissues. The IHC result is usually observed with a microscope. The method has high specificity and reproducibility but is prone to inter-observer variability, and only one marker can be detected in each assay, thus is not suitable for detecting multiple markers.
- ICC (Immunocytochemistry) is also an immune-based staining technique similar to IHC but is used for staining cell samples, like cell smears, cell blocks or liquid-based monolayers. The fixation method of ICC is different from that of IHC, often involving wet fixation or air drying. Another difference is that it is usually more challenging to get reliable positive or negative control samples to interpret the ICC results. In summary, although the principle and application of ICC and IHC are similar, ICC is more suitable for cell-based samples, and IHC is more suitable for tissue sections.
- IF (Immunofluorescence) is an immune-detection technique using fluorescent labeling, in which fluorescent dyes are conjugated to antibodies to visualize the expression of antigens in cells and tissues. It offers high sensitivity and a broad dynamic range, enabling the simultaneous detection of multiple markers. This makes it ideal for quantitative analysis and spatial distribution studies. IF is commonly used to investigate cellular functions, intercellular interactions, and the tumor microenvironment (TME).
Which Technique is Best for Your Experiment?
| Technique | Sample Types | Typical Applications |
|---|---|---|
| IHC |
|
|
| ICC |
|
|
| IF |
|
|
Comparison of Advantages and Disadvantages
| Technique | Advantages | Disadvantages |
|---|---|---|
| IHC |
|
|
| ICC |
|
|
| IF |
|
|
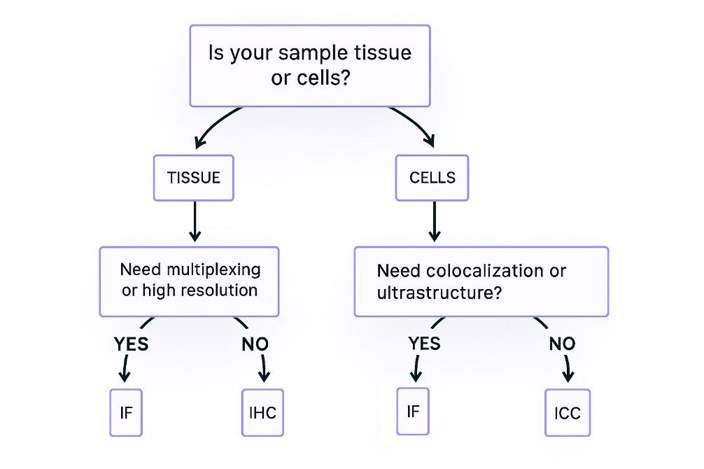
Still undecided? Try this universal strategy:
Start with ICC (chromogenic method) → Rapidly validate antibody efficacy.
Optimize and upgrade to IF → Obtain publication-quality images.
Supplement with IHC for clinical samples → Meet pathological diagnostic requirements.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
|---|---|
| Immunohistochemistry (IHC), Immunofluorescence (IF) Service | Creative Bioarray offers a comprehensive IHC service from project design, marker selection to image completion and data analysis. |
| Histology Services | Creative Bioarray offers tissue processing, embedding, sectioning, and staining, along with set of histological examination services. |
Reference
- Gheban-Roșca I-A, Gheban B-A, et al. Immunohistochemical and Morphometric Analysis of Lung Tissue in Fatal COVID-19. Diagnostics. 2024; 14(9):914.