CFU Assay for Hematopoietic Cell
The colony-forming cell (CFC) assay, also known as the colony-forming unit (CFU) assay, is currently the gold standard for in vitro testing of hematopoietic stem/progenitor cell function.
CFU assay is usually performed by inoculating hematopoietic cells at a certain density in a semi-solid culture medium with appropriate cell factors. Hematopoietic progenitor cells will proliferate and differentiate to form mature hematopoietic cell colonies during the incubation period, and then the function and quality of hematopoietic cells will be identified based on the number and species of the colonies, which are judged based on the criteria of morphology and cellular phenotype.
CFU colonies are generally categorized into red lineage colony forming units (CFU-E), bursting red cell colony forming units (BFU-E), granulocyte colony forming units (CFU-G), macrophage colony forming units (CFU-M), granulocyte/macrophage (CFU-GM) and mixed cell lineage colony forming units (CFU-GEMM).
Hematopoietic Cell CFU Colony Assay
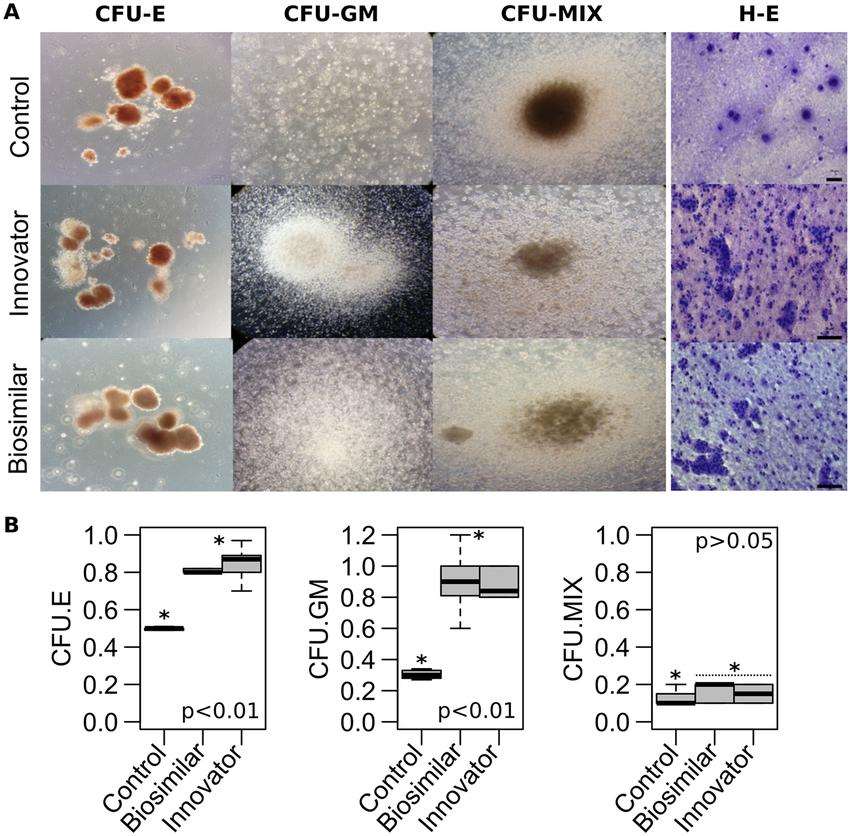 Fig. 1 Hematopoietic colony-forming cell assays. (Avila-Portillo LM, et al., 2020)
Fig. 1 Hematopoietic colony-forming cell assays. (Avila-Portillo LM, et al., 2020)
- Human samples
The CFU assay for hematopoietic cells in human samples provides a powerful tool for studying various disorders of the blood and bone marrow, such as leukemia, myelodysplastic syndromes, and aplastic anemia. By isolating and culturing hematopoietic cells in a semi-solid medium, individual colonies derived from single precursor cells can be identified and quantified. These colonies represent the clonal expansion and differentiation potential of hematopoietic progenitor cells. - Mouse samples
In addition to human samples, the CFU assay for hematopoietic cells is widely employed in murine models as well. Mouse models provide a valuable platform for studying hematopoiesis and assessing the effects of genetic modifications or intervention strategies on hematopoietic cell function.
Hematopoietic Stem Cell CFU Colony Assay Procedure
- Harvesting and isolation of hematopoietic cells
Hematopoietic cells can be isolated from bone marrow, peripheral blood, or umbilical cord blood, depending on the sample source. Various techniques such as density gradient centrifugation or magnetic cell sorting can be employed for cell isolation. - Plating the cells
The isolated hematopoietic cells are mixed with a semi-solid culture medium containing cytokines and growth factors that support colony formation and differentiation. The cell-medium mixture is then plated onto suitable culture dishes or plates. - Incubation
The plated cells are incubated under controlled conditions to promote colony growth. The duration of incubation varies depending on the type of cells and experimental requirements. - Colony identification and quantification
After the incubation period, individual colonies formed by clonally derived cells can be visually identified and counted. Various staining techniques can be employed to aid in colony visualization and characterization.
Precautions for CFU Colony Assay
- The quality and viability of the isolated hematopoietic cells are critical for obtaining meaningful results. Proper sample processing and storage conditions should be maintained to prevent cell damage or loss of functionality.
- The choice of culture medium, cytokines, and growth factors can significantly impact colony formation and differentiation. It is important to optimize these conditions to ensure optimal colony growth and accurate assessment of cell potential.
- Care should be taken to accurately identify and count individual colonies. Using appropriate staining methods and trained personnel can help minimize errors and variability in colony quantification.
Creative Bioarray Relevant Recommendations
- The CFU assay for hematopoietic cells is a valuable tool for studying the clonogenic potential and differentiation capacities of these cells. It has wide-ranging applications in both human samples and animal models, offering insights into hematopoietic development and disease states.
- Creative Bioarray provides high-quality human hematopoietic stem cells and corresponding kits, including Human Hematopoietic Stem Cells, Human Hematopoietic Expansion Medium, Human Hematopoietic Growth Medium, Human Hematopoietic Stem Cell Growth Medium, and Hematopoietic Progenitor Medium Kit, to accelerate our customers' scientific research.
Reference
- Avila-Portillo LM, et al. (2020). "Comparative Analysis of the Biosimilar and Innovative G-CSF Modulated Pathways on Umbilical Cord Blood-Derived Mononuclear Cells." Bioinform Biol Insights. 14, 1177932220913307.