Acute Liver Injury Models
Various factors can cause acute liver injury, and most factors are caused by drug-induced liver toxicity. Despite the progress made in this area, the management of acute liver injury remains a significant challenge in clinical medicine. The availability of sufficient experimental models is essential to understand this situation better and allow the identification of new drug targets, test the efficacy of new therapeutic interventions, and serve as a model for evaluating toxicity mechanisms.
Creative Bioarray provides various pharmacological models of acute liver injury, including models induced by carbon tetrachloride(CCl4), acetaminophen(APAP), thioacetamide(TAA), D-Galactosamine(D-GalN), lipopolysaccharide(LPS), concanavalin A(ConA) or Fas Ab.
Acute Liver Injury Assays
- Carbon tetrachloride(CCl4)
CCl4 is a well-known hepatotoxin widely used to induce acute and chronic toxic liver injury in a wide range of laboratory animals. The toxicity of CCl4 results from its reductive dehalogenation by cytochrome P450 into the highly reactive free radical trichloromethyl radical (NCCl3). Free radicals probably activateKupffer cells and mediate the hepatic inflammation process through producing TNF-α and other pro-inflammatory cytokines. In the presence of excess oxygen, NCCl3 can transform into trichloromethyl peroxy radical CCl3OON, another highly reactive species.
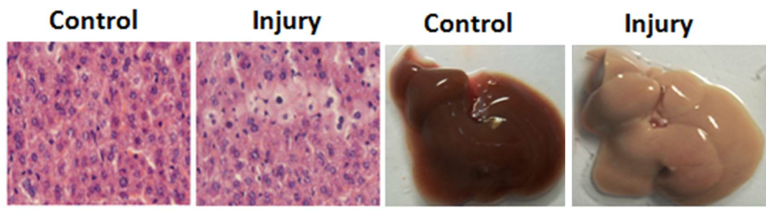 Figure 1. Acute liver injury induced by CCl4(F. Zhang et al., 2014).
Figure 1. Acute liver injury induced by CCl4(F. Zhang et al., 2014).
- Acetaminophen(APAP)
The metabolism of APAP occurs in the liver. Under normal circumstances, the drug undergoes biotransformation through a combination of glucuronidation and sulfation and is then excreted by the kidneys. In excess, these pathways are saturated, and the P450 cytochrome oxidase system metabolizes APAP. This leads to the formation of the toxic electrophile N-acetyl-p-benzoquinoneimine (NAPQI), which can cause cell damage unless combined with endogenous glutathione. NAPQI is believed to interrupt the mitochondrial calcium flux that causes cell damage by forming oxygen free radicals, hydroxyl free radicals, nitrites, and nitrates. The cascade is amplified by the activation of Kupffer cells and the production of cytotoxic mediators (such as cytokines and free radicals), leading to apoptosis and cell necrosis.
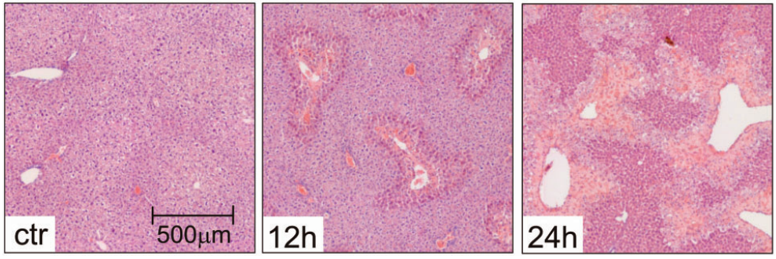 Figure 2. Livers from control mice (ctr, treated with NaCl 0.9%) and APAP-treated mice stained with H&E show progressing necrotic areas (lighter), 12 h and 24 h after APAP overdose(Mossanen & Tacke, 2015).
Figure 2. Livers from control mice (ctr, treated with NaCl 0.9%) and APAP-treated mice stained with H&E show progressing necrotic areas (lighter), 12 h and 24 h after APAP overdose(Mossanen & Tacke, 2015).
- Thioacetamide(TAA)
For many years, thioacetamide (TAA) has also been used to induce acute liver injury models. After TAA is bioconverted into active metabolites through the flavin adenine dinucleotide monooxygenase pathway, it causes necrosis of liver cells, thereby forming TAA-S-oxide. It is believed that this can cause apoptosis; however, as the dose increases, electrophiles and free radicals are released, causing lipid peroxidation and lobular center necrosis.
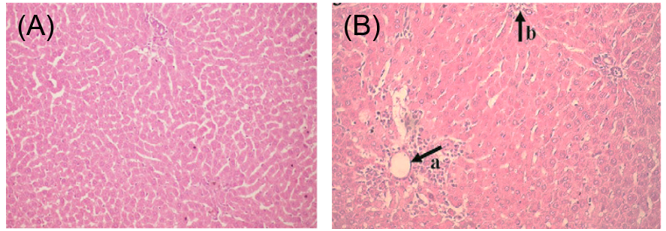 Figure 3. The representative hepatic histopathology: (A) normal rat liver; (B) centrilobular necrosis and infiltration in thioacetamide (TAA)-treated rats;
Figure 3. The representative hepatic histopathology: (A) normal rat liver; (B) centrilobular necrosis and infiltration in thioacetamide (TAA)-treated rats;
- D-Galactosamine(D-GalN)/LPS
D-GalN acts as a sensitizing agent because it depletes hepatic stores of uridine triphosphate via the galactose pathway. Low doses of LPS cause modest inflammatory responses resulting in increased susceptibility to numerous hepatotoxic chemicals combined with D-GalN. As a response, hepatocytes strive to clear LPS, followed by an inflammatory response, oxidative stress, and even hepatic necrosis.
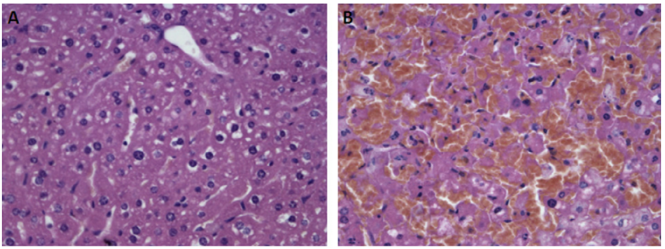 Figure 4. Histology of liver sections. (A): Control group treated with PBS. (B): Group treated with LPS/D-GalN(L. Zhang et al., 2010).
Figure 4. Histology of liver sections. (A): Control group treated with PBS. (B): Group treated with LPS/D-GalN(L. Zhang et al., 2010).
- Concanavalin A(ConA)
ConA is a bean-derived lectin, which induces T-cell-mediated liver damage dependent mainly on CD4+ T-cells. The early binding of ConA with sinusoidal endothelial cells recruits CD4+ T cells, which are the primary source of IFN-γ, but also contribute to the formation of TNF-α and other cytokines. Both TNF-α and IFN-γ are critical mediators of ConA-induced cytotoxicity. TNF-α and IFN-γ can synergistically activate a number of adhesion molecules on sinusoidal endothelial cells and Kupffer cells, as well as chemokines in hepatocytes, recruiting neutrophils. Neutrophils contribute to ConA-induced liver injury by direct cytotoxicity and indirectly also by promoting T-cell recruitment.
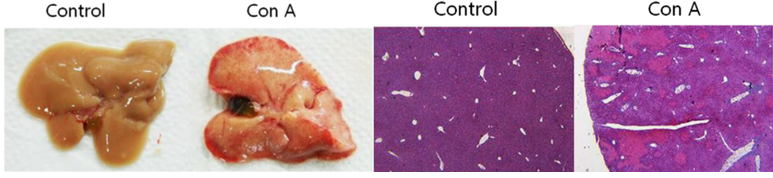 Figure 5. Acute lung injury induced by ConA(Ryu et al., 2014).
Figure 5. Acute lung injury induced by ConA(Ryu et al., 2014).
- Fas
The Fas receptor is a member of the TNF receptor family with a death domain that can assemble a death-inducing signaling complex to induce caspase activation and apoptosis. The agonistic Fas antibody Jo2 can dose-dependently induce apoptosis in both hepatocytes and sinusoidal endothelial cells. Fas ligand FasL or its antibody Jo2 is often used in research to induce liver injury models.
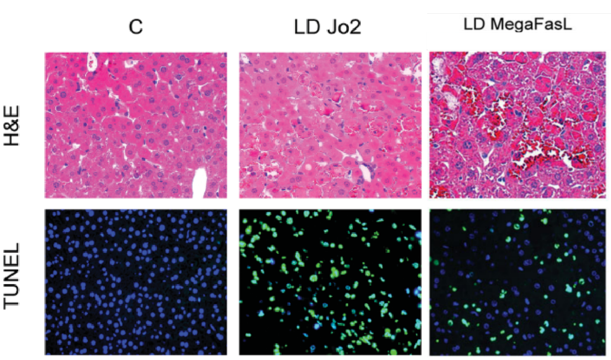 Figure 6. Acute liver injury induced Jo2 and FasL(Schüngel et al., 2009).
Figure 6. Acute liver injury induced Jo2 and FasL(Schüngel et al., 2009).
Available Assays
- Cytotoxicity assays
- Measurement of cytokines
- Flow cytometric analysis of cell death
- Measurement of serum liver enzymes
- Hematoxylin and eosin staining (H&E)
- Immunohistochemistry
- Quantitative real-time PCR
- TUNEL stain
- Western blot analysis
- Gene expression analysis
Applications
- To evaluate the preventive and therapeutic effects of drugs on the occurrence and development of diseases.
- To explore the mechanism of drug action.
- To verify the function of new genes
Quotation and ordering
If you have any special needs or questions regarding our services, please feel free to contact us. We look forward to cooperating with you in the future.
References
- Mossanen, J.; Tacke, F. Acetaminophen-induced acute liver injury in mice. Laboratory Animals, (2015), 49(1_suppl), 30-36.
- Ryu, K.-H.; et al. Tonsil-derived mesenchymal stem cells alleviate concanavalin A-induced acute liver injury. Experimental Cell Research, (2014), 326(1), 143-154.
- Schüngel, S.; et al. The strength of the Fas ligand signal determines whether hepatocytes act as type 1 or type 2 cells in murine livers. Hepatology, (2009), 50(5), 1558-1566.
- Zhang, F.; et al. The Protective Effect of Esculentoside A on Experimental Acute Liver Injury in Mice. PLOS ONE, (2014), 9(11), e113107.
- Zhang, L., et al. Protective effects of Asiaticoside on acute liver injury induced by lipopolysaccharide/D-galactosamine in mice. Phytomedicine, (2010), 17(10), 811-819.