Why Cardiotoxicity Matters in R&D?
Cardiotoxicity is one of the most common causes of drug attrition during late-stage development and post-market withdrawal. Despite advancements in preclinical safety evaluation, a large number of compounds with high target engagement are abandoned due to unpredicted cardiotoxicity. The heart is highly sensitive to molecular perturbations, and even minor off-target interactions can result in arrhythmias, contractile dysfunction, or irreversible myocardial injury.
Pharmaceutical companies face enormous pressure to minimize these risks. Unfortunately, routine toxicology assessments may miss early or subtle cardiac safety signals. For example, typical in vitro and in vivo assays are not always sensitive to ion channel interference, metabolic stress, or structural remodeling that may only become evident during advanced testing or in clinical trials. As a result, there is a need for improved understanding of cardiotoxicity and more predictive detection platforms to improve drug safety and avoid late-stage attrition.
What Is Cardiotoxicity?
Cardiotoxicity is an umbrella term that refers to the adverse effect of a pharmaceutical compound or environmental toxin on the structure and function of the heart. It is a complex, multi-factorial process that can present in various forms, from subtle electrophysiological changes and impaired contractility to structural remodeling and irreversible myocardial damage.
Clinically, cardiotoxicity may present in different forms:
- Electrical dysfunction, such as QT interval prolongation and arrhythmias, often caused by unintended inhibition of the hERG potassium channel.
- Functional impairment, such as decreased left ventricular ejection fraction (LVEF), a common endpoint in both preclinical and clinical studies to measure contractile function.
- Structural damage, as evidenced by histopathological changes, fibrosis, or ultrastructural mitochondrial injury.
- Biochemical markers of myocardial stress or injury, such as elevated cardiac enzymes (e.g., troponins, creatine kinase-MB).
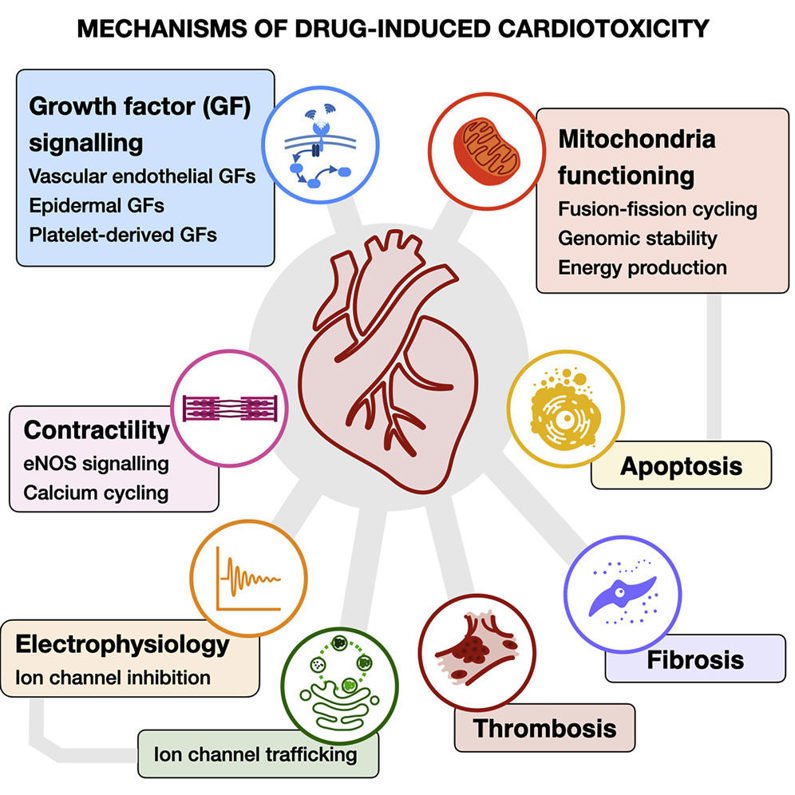
Mechanisms of Drug-Induced Cardiotoxicity
The pathogenesis of cardiotoxicity is multifaceted. Several well-characterized mechanisms contribute to cardiac injury:
1. Ion Channel Interference
The most common and well-known mechanism of cardiotoxicity is the interference with ion channel activity. In particular, drugs that block the hERG potassium channel cause QT interval prolongation on the electrocardiogram, making patients susceptible to torsades de pointes and fatal arrhythmias. Accordingly, hERG screening is now required for most new chemical entities.
2. Mitochondrial Dysfunction
The heart has exceptionally high energy demands, and mitochondrial integrity is crucial for sustaining contractility. Some drugs interfere with oxidative phosphorylation or increase mitochondrial permeability, resulting in lower ATP production and an overall "cardiac energy crisis." Chronic energy depletion ultimately leads to cardiomyocyte apoptosis and heart failure.
3. Oxidative Stress and Fibrosis
Some compounds promote excessive production of reactive oxygen species (ROS), overwhelming antioxidant defenses. In addition to directly damaging lipids, proteins, and DNA, oxidative stress also induces pro-fibrotic signaling pathways. Cardiac fibrosis stiffens the ventricular wall, impairs diastolic filling, and causes arrhythmias.
4. Other Mechanisms
Additional mechanisms include altered calcium homeostasis, immune-mediated myocarditis, and direct structural damage to sarcomeric proteins. The diversity of injury pathways highlights the need for multiparametric safety assessment rather than reliance on a single biomarker.
High-Risk Drug Classes
| Drug Class | Cardiotoxic Effects | Examples |
|---|---|---|
| Chemotherapy Drugs | Myocardial damage, cardiomyopathy, arrhythmias, heart failure | Anthracyclines (e.g., Doxorubicin, Daunorubicin), Alkylating agents (e.g., Cyclophosphamide), Antimetabolites (e.g., 5-Fluorouracil), Antimitotic agents (e.g., Paclitaxel) |
| Antiarrhythmic Drugs | Arrhythmias, QT prolongation, conduction abnormalities | Amiodarone, Macrolides (e.g., Clarithromycin, Azithromycin), Quinolones (e.g., Ciprofloxacin, Levofloxacin) |
| Other Drugs | Cardiotoxicity, QT prolongation, conduction abnormalities | Beta-2 agonists, Antihistamines, Antivirals |
| Anticancer Drugs (e.g., HER2-targeted therapies, Checkpoint inhibitors) | Cardiomyopathy, heart failure, arrhythmias, QT prolongation | Trastuzumab (Herceptin), Immune checkpoint inhibitors, Tyrosine kinase inhibitors (TKIs) |
| Other Cardiac-Toxic Drugs | Bradycardia, tachycardia, hypotension, hypertension, QRS prolongation, QTc prolongation | Amiodarone, Beta-blockers, Antipsychotics, Cocaine, Digoxin |
Key Considerations During Drug Development
Given the breadth of cardiotoxic risks, developers must adopt proactive strategies throughout the pipeline:
Early Risk Identification
Integrating predictive assays such as hERG screening, CiPA protocols, and human iPSC-derived cardiomyocyte models early in the drug discovery process helps to identify potential ion channel liabilities or mitochondrial toxicity before they become more costly and challenging to address later.
Comprehensive Safety Profiling
Relying solely on QT prolongation is insufficient. Developers should evaluate LVEF, biomarkers (troponins, cardiac enzymes), and imaging-based endpoints to capture both acute and chronic cardiac injury.
Patient-Specific Risk Factors
Factoring in genetic predispositions (e.g., congenital long QT syndrome), comorbidities (hypertension, diabetes), and potential drug-drug interactions with common co-medications can help to better understand and mitigate drug-induced cardiotoxicity risks in specific patient populations.
Benefit–Risk Balancing
In cases where high-risk drugs are being developed for high-benefit applications, such as anticancer therapies, incorporating cardiotoxicity management strategies like dose adjustments, cardioprotective co-therapies, or more intensive cardiac monitoring into the trial design from the outset can help to manage these risks proactively.
Regulatory Alignment
Adhering to international guidelines (ICH S7B, E14) and newer frameworks like CiPA ensures compliance and increases the credibility of cardiac safety packages submitted for approval.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
|---|---|
| In Vitro Cardiotoxicity | Through our comprehensive and detailed assessment methods, Creative Bioarray helps reduce the cardiac safety risks of your drugs, thereby minimizing the potential for development failure and associated economic losses. |
| hERG Safety Assay | Learn more about our hERG Safety Assay service for cardiotoxicity assessment. |
| QT Prolongation Assay | Creative Bioarray offers in vitro QT prolongation using human cardiomyocytes to evaluate the cardiotoxicity of compounds with QT interval as the indicator. |
| 3D Cardiovascular Toxicity Service | Creative-Bioarray offers the opportunity for highly predictable pre-clinical cardio-toxicity testing to help our customs to reduce both the cost and duration of bringing a new drug candidate to market |
Reference
- Mamoshina P, Rodriguez B, et al. Toward a broader view of mechanisms of drug cardiotoxicity. Cell Rep Med. 2021. 2(3):100216.