Radiation-Induced Intestinal Injury Model
Creative Bioarray offers an experimental intestinal injury model induced by radiation, which serves as an invaluable animal model for investigating the pathogenesis, prevention, and treatment of radiation-induced intestinal injury. This model is crucial for understanding the mechanisms underlying radiation damage to the intestinal tract, which is a common and serious complication of radiation therapy for abdominal and pelvic cancers. By utilizing this model, researchers can explore the effects of radiation on the intestinal mucosa, including changes in cell proliferation, apoptosis, and the inflammatory response. Additionally, this model facilitates the development and testing of novel therapeutic strategies aimed at mitigating the adverse effects of radiation on the intestines, thereby improving the quality of life for cancer patients undergoing treatment.
The small intestine is highly sensitive to ionizing radiation, which presents significant challenges in the treatment of abdominal or pelvic tumors with radiation therapy. This sensitivity results in radiation-induced intestinal damage, which is characterized by symptoms like anorexia, vomiting, diarrhea, dehydration, systemic infection, and, in severe cases, septic shock and death. Ionizing radiation directly affects the DNA, lipids, and proteins in both cancerous and non-cancerous cells within the gastrointestinal tract during radiation therapy. Unfortunately, there is currently no specific treatment available for radiation enteritis in clinical settings, greatly impacting the quality of life for patients. Therefore, the development of effective protectors against radiation-induced intestinal damage remains a critical focus in radiation protection research.
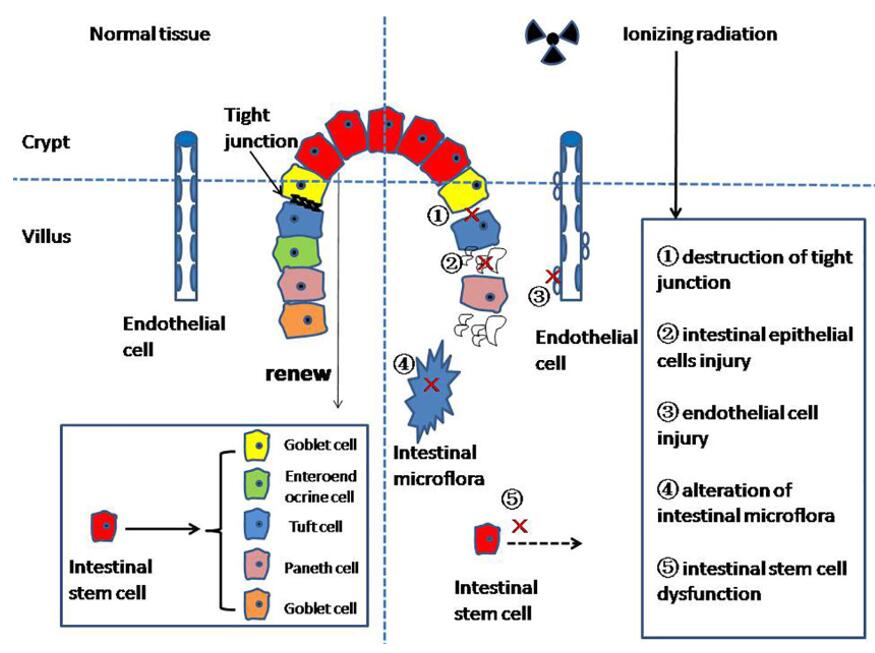 Fig. 1 The mechanism of radiation enteritis. (Fan et al. 2022)
Fig. 1 The mechanism of radiation enteritis. (Fan et al. 2022)
Our Radiation-Induced Intestinal Injury Model
- Animal Species
Mouse
- Modeling Method
Mice are treated with an X-ray radiation dose of 12 Gy to induce intestinal injury.
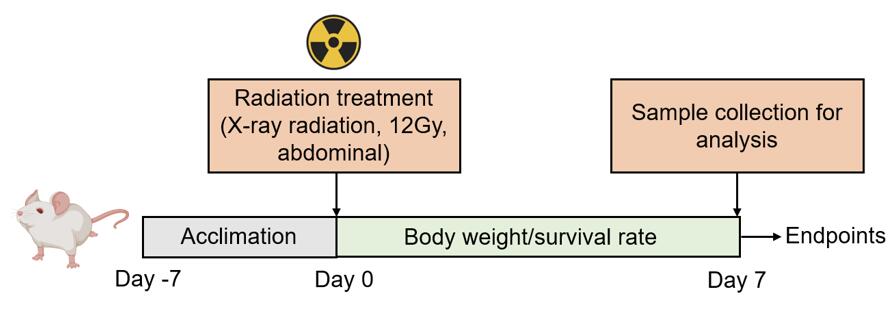 Fig. 2 Modeling method of radiation-induced intestinal injury.
Fig. 2 Modeling method of radiation-induced intestinal injury.
- Endpoints
- Survival rate
- Body weight
- Histology analysis
- qPCR or Western blot
- Other customized endpoints
Example Data
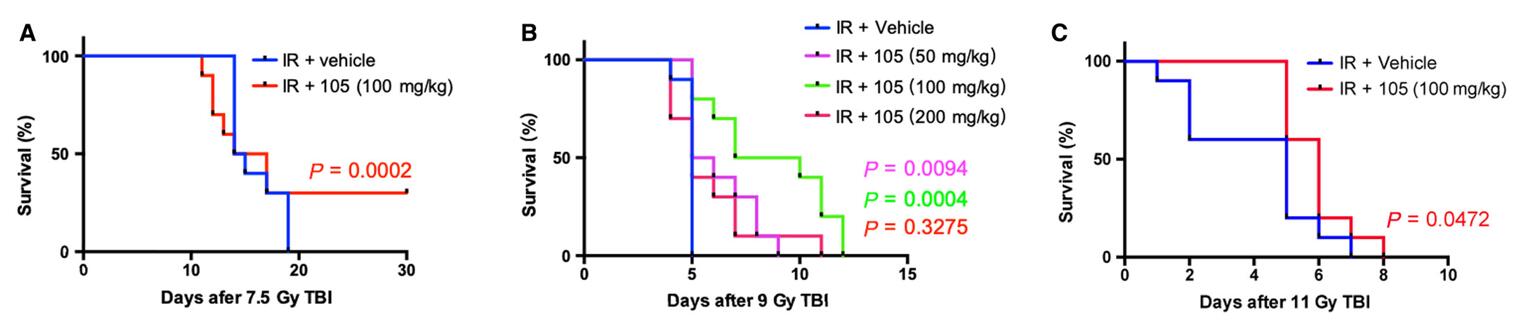 Fig. 3 XH-105 improves the survival of mice after 9.0 Gy total body irradiation (TBI). Kaplan-Meier survival analysis of mice exposed to 7.5, 9.0 or 11.0 Gy TBI. A, 100 mg/kg XH-105 treated mice have 30% survival beyond 30 d post 7.5 Gy TBI, compared with IR mice with 100% mortality within 20 d of radiation exposure. B, three doses (50, 100, 200 mg/kg) of XH-105 treated mice show reduced mortality following lethal doses of TBI (9.0 Gy) within 13 d, compare with IR group 100% mortality within 5 d. C, The Kaplan-Meier survival curve of vehicle and 100 mg/kg XH-105 treated mice after 11.0 Gy TBI. (Cheng et al. 2019)
Fig. 3 XH-105 improves the survival of mice after 9.0 Gy total body irradiation (TBI). Kaplan-Meier survival analysis of mice exposed to 7.5, 9.0 or 11.0 Gy TBI. A, 100 mg/kg XH-105 treated mice have 30% survival beyond 30 d post 7.5 Gy TBI, compared with IR mice with 100% mortality within 20 d of radiation exposure. B, three doses (50, 100, 200 mg/kg) of XH-105 treated mice show reduced mortality following lethal doses of TBI (9.0 Gy) within 13 d, compare with IR group 100% mortality within 5 d. C, The Kaplan-Meier survival curve of vehicle and 100 mg/kg XH-105 treated mice after 11.0 Gy TBI. (Cheng et al. 2019)
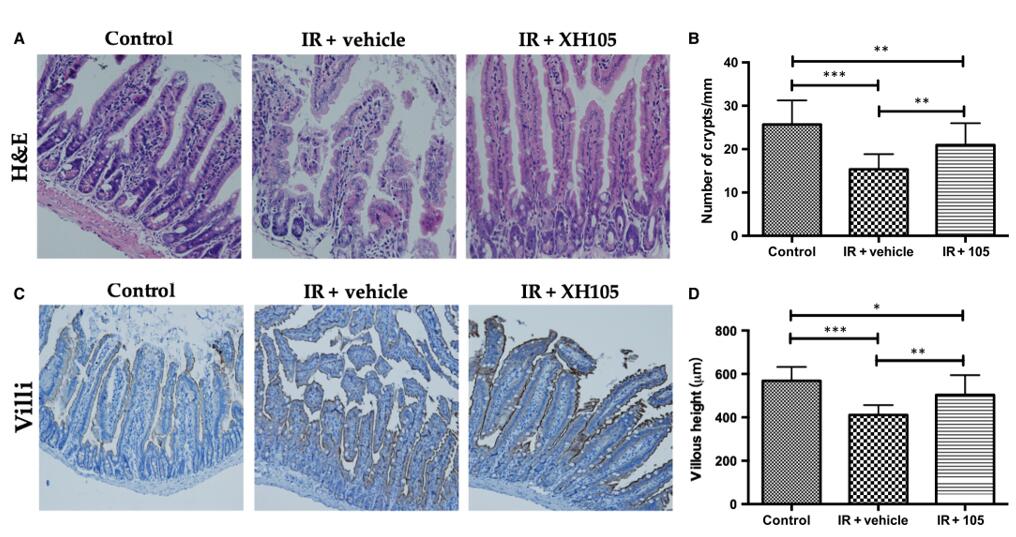 Fig. 4 XH-105 protects the intestinal morphology of mice after 9.0 Gy total body irradiation (TBI). A, Representative images showing the structure in cross-sections of the small intestine with haematoxylin-eosin (H&E) stained. B, Histogram showing the number of crypts. C, Immunohistochemistry images showing the expression of Villi. D, Histogram demonstrating villus length in intestinal section from the control group, vehicle-treated group and XH-105-treated group. (Cheng et al. 2019)
Fig. 4 XH-105 protects the intestinal morphology of mice after 9.0 Gy total body irradiation (TBI). A, Representative images showing the structure in cross-sections of the small intestine with haematoxylin-eosin (H&E) stained. B, Histogram showing the number of crypts. C, Immunohistochemistry images showing the expression of Villi. D, Histogram demonstrating villus length in intestinal section from the control group, vehicle-treated group and XH-105-treated group. (Cheng et al. 2019)
Quotation and Ordering
Creative Bioarray assists clients in selecting the most validated and reliable models for testing their compounds, ensuring the acquisition of clinically relevant data. If you are interested in our services, please feel free to contact us at any time or submit an inquiry to us directly.
References
- Cheng, Y., et al. The protective effects of XH‐105 against radiation‐induced intestinal injury. Journal of cellular and molecular medicine, 2019, 23(3): 2238-2247.
- Fan, J., et al. Research progress on the mechanism of radiation enteritis. Frontiers in Oncology, 2022, 12: 888962.