Frequently Asked Questions
Tissue Samples FAQ
- Q1: Where do the tissues come from?
A: All of the tissues in our Tissue bank come from hospitals and clinics in the U.S. However, we do have international clinic partners that can be access when necessary.
- Q2: How long are tissues fixed after harvest from the body, either through biopsy or autopsy?
A: 24-48 hours.
- Q3: What is the time between tissue collection and fixation for surgical cases?
A: After surgery, tissue samples are put into formalin and brought to the pathology department of our collaborating hospitals. This process occurs less than 10 minutes after surgery and before the fixation.
- Q4: What is the post-mortem interval to fixation for autopsy samples?
A: For post mortem tissues, the typical time in formalin fixative depends on the duration of procedures performed after death, including the acquisition of consent from the family. Nevertheless, the post mortem interval is less than six hours.
- Q5: What information do you provide for FFPE blocks?
A: Age, Sex, Race, Histological diagnosis, Differentiation grade, TNM, Stage, Tumor size, Date of surgery, etc.
- Q6: How long can paraffin tissue sections be stored?
A: We have tested that the antigenicity was not lost (no significant difference in statistics) with paraffin tissue sections kept in a refrigerator (4°C) dated 4 years ago.
- Q7: What is the appropriate size of the tissue inside the paraffin tissue blocks?
A: Standard sample size is 0.5*1*1 cm.
- Q8: What is fresh frozen tissue sample size?
A: Standard sample size is 0.5-1 gram. Tissue samples of 2-3 gram are available upon request.
- Q9: What is the time between surgery/biopsy and tissue samples freezing?
A: All fresh tissue samples are collected by certified medical pathologists and are snap-frozen in liquid nitrogen within 30 minutes of surgery excision.
- Q10: How are the samples shipped?
A: Frozen: All frozen shipments are shipped on dry ice.
FFPE & TMA: FFPE specimen and TMA’s are packaged in slide cases and clam shells for protection.
Primary Cells FAQ
- Q1: What is the difference between “primary cells” and “cell lines”?
A: Primary cells are cells taken directly from living tissue and established for growth in vitro. Primary cells have a finite lifespan and are widely used because they retain many of the markers seen in vivo. Primary cells from different species allow you to highlight potential differences between humans and preclinical test species. Before in vivo studies, using primary cells can refine doses and reduce the number of animals required for preclinical toxicology. Human primary cells can be used to determine accuracy of extrapolating human data from an animal model.
A cell line is a population of cells that has undergone a genetic transformation, resulting in indefinite growth potential. In practice, cell lines can be cultured through a very high number of subcultures, although some further genotypic, and therefore phenotypic, changes may occur at very high passage numbers.
Generally, cell lines lack many of the markers seen in vivo and also show very different marker profiles than primary cells.
- Q2: How should I deal with cryopreserved cells upon receiving?
A: Upon receiving the package, cryopreserved cells should be immediately transferred from the dry ice shipping container to a liquid nitrogen storage tank in order to prevent cells from thawing.
In the uncertain event that no dry ice is left in the package upon receiving, thaw and use the cells immediately.
Do not store the cells at -20°C or -80°C as this causes irreversible damage to the cells.
- Q3: How do I establish a culture from cryopreserved cells?
A: Note: Please refer to the cell product sheet for detailed instructions.
- Remove a vial of cells from liquid nitrogen, taking care to protect hands and eyes.
- Loosen the cap on the vial ¼ turn for 10 seconds to release any liquid nitrogen that may be trapped in the threads, then re-tighten the cap.
- Place the frozen vial in a 37oC water bath. Hold and rotate the vial gently until the contents completely thaw.
- Remove the vial from the water bath promptly, wipe it down with 70% ethanol and transfer it to the sterile field.
- Remove the cap carefully without touching the interior threads. Gently resuspend the contents.
- Take 20 μL from the vial and dilute the cell suspension in 20 μL of trypan blue solution.
- Use a hemacytometer to determine the number of viable cells per mL.
- Dilute the contents of the vial (1 mL) to the concentration recommended by the product instructions.
- Add 5 mL of cell suspension to each 25 cm² culture flask or 15 mL of cell suspension to each 75 cm² culture flask. Gently rock the vessel to distribute the cells evenly.
- Return the culture vessel to the incubator (37°C, 5% CO2/95% air).
- For the best results, do not disturb the culture for at least 24 hours after the culture has been initiated. Refresh culture medium the next day to remove the residual DMSO and unattached cells.
- Q4: How do I calculate the number of flasks or plates I should set up for culturing the cells?
A: The following equations can be used to determine the maximum number of culture vessels that can be set up:
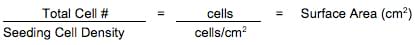
The effective growth areas for common tissue culture ware are listed below:
Culture FlasksT-25T-75T-150T-250Growth Area25 cm275 cm2150 cm2250 cm2Culture Dishes35 mm60 mm100 mm150 mmGrowth Area9.6 cm220.4 cm257 cm2143 cm2Multiwell Plates6 well12 well24 well96 wellGrowth Area9.6 cm23.5 cm21.9 cm20.33 cm2
- Q5: How often should I change the cell culture medium on my primary cells?
A: After thawing and plating the cryopreserved cells, the first medium change should be done after 24 hours or overnight, so that both residual DMSO and any dead cells are removed. In general, the medium should be changed every 2-3 days hours depending on the confluency of the cells until the cells are ready to be passaged.
- Q6: How many passages can I obtain with primary cells?
A: We uses the term "population doubling" instead of "passage" for describing the growth potential of the cells. A population doubling is a two-fold increase in the total number of cells in culture. The term passage number refers to the number of times that a cell population has been removed from the culture vessel and undergone a subculture (passage) process. Because the split ratios varies in different researchers when they subculture, it is difficult to predict how many passages can be obtained with a particular primary cell. Our primary cells are guaranteed for a specified number of population doublings on the cell product sheet.
- Q7: Can I expand primary cells and re-freeze them?
A: We do not recommend our customers refreeze primary cells. The cryopreservation process may result in altered growth performance of the cells.
- Q8: There are visible flocculants in my FBS – is this normal?
A: The presence of flocculants in FBS is due to lipoprotein precipitates – these are normal and are not harmful to cells. Customers can centrifuge briefly at 400g and take the supernatant to remove the precipitates, though it is not necessary.
- Q9: Do I need to spin the cells out of the cryopreservation medium before plating?
A: We do not recommend to spin cells out of cryopreservation medium before plating. Centrifugation can be harmful to cells, particularly if inappropriately high speeds are used. DMSO will be sufficiently diluted in medium when you plate the cells. For example, if you plate the whole cryovial (one milliliter) of cells in a T-75 flask with 15 ml of medium, the DMSO concentration will be less than 0.67% (v/v).Therefore, our product instructions include a detailed protocol which involves diluting the cells into culture medium at the recommended seeding density and volume of medium.
Stem Cells FAQ
- Q1: What is a stem cell?
A: Stem cells are a class of undifferentiated cells that have the remarkable potential to develop into many different cell types in the body. A stem cell is essentially a “blank” cell, capable of becoming another more differentiated cell type in the body with a more specialized function, such as a skin cell, a muscle cell, or a nerve cell. There are three classes of stem cells: totipotent, multipotent, and pluripotent.
- Q2: How many times can stem cells be passaged?
A: It may vary from different types of stem cells. For pluripotent stem cells which are derived from the inner cell mass of blastocysts or induced by transcription factors from somatic cells, hPSC can self-renew indefinitely. Stem cells or progenitor cells derived from adult tissues usually have limited self-renewal and differentiate eventually, such as neural progenitors. Our human mesenchymal stem cells are guaranteed for 15 population doublings.
- Q3: Can hPSC-derived cardiomyocytes be expanded, passaged and cryopreserved?
A: Expanding, passaging, or re-freezing hPSC-derived cardiomyocytes are not recommended.
- Q4: How do embryonic stem cells, somatic stem cells, and cord blood stem cells differ?
A: Embryonic stem cells (ESCs) are derived from the embryo and have the potential to become all the different cell types of the body (pluripotency). Somatic stem cells, sometimes called adult stem cells, are found in organs or tissues, can self-renew and yield the differentiated cell types comprising that organ or tissue (multipotency), and are important for maintenance and repair of the organ or tissue. Cord blood stem cells can be isolated from the umbilical cord of newborn infants and are less mature than adult stem cells. Cord blood stem cells are a type of somatic stem cell. Somatic stem cells are restricted in the types of cells they can produce in the lab.
FISH FAQ
- Q1: Why we should use ISH(in situ hybridization)?
A: In situ hybridization indicates the localization of gene ex pression in their cellular environment. A labeled RNA or DNA probe can be used to hybridize to a known target mRNA or DNA sequence within a sample. This labeled RNA or DNA probe can then be detected by using an antibody to detect the label on the probe. The probes can therefore be used to detect ex pression of a gene of interest and the location of the mRNA.
- Q2: What is BAC and PAC clones?
A: Bacterial artificial chromosome (BAC) and P1-derived artificial chromosome (PAC) cloning systems, developed in the 1990s, are ideal cloning systems for carrying DNA fragments 100 to 300 kb in size. These libraries, including the RPCI-11 human female library and RPCI-23 mouse male library, are amenable to genomic analysis, since their DNAs are easily prepared and manipulated.
- Q3: How should we store general sample when using frozen sections?
A: For good results on older slides, the slides should not be stored dry at room temperature. They should be stored either in 100% ethanol at -20°C, or in a plastic box covered in saran wrap at -20°C or -80°C. Slides stored in this way can be used for several years.
- Q4: What is the difference between RNA probes and DNA probes?
A: RNA probes should be between 250 to 1500 bases in length. Probes approximately 800 bases long exhibit the highest sensitivity and specificity. Ideally transcription templates should allow for transcription of both probe (antisense strand) and negative control (sense strand) RNAs. Cloning into a vector with opposable promoters will achieve this. Circular templates must be linearized before making a probe. PCR templates can also be used for this purpose. DNA probes can also provide high sensitivity for in situ hybridization.. However, they do not hybridize as strongly to the target mRNA molecules as RNA probes. Therefore, formaldehyde should not be used in the post hybridization washes when using DNA probes.
Karyotyping (G-Banded) FAQ
- Q1: When do we need to do karyotyping analysis?
A: Karyotyping analysis is used as a baseline screen:
- when cell lines are derived
- before the start of an experiment
- at the conclusion of an experiment
- before publication
- as part of routine cell line QC
- as part of cell banking QC
- when cultures show unusual growth properties
- Q2: What do karyotyping results show?
A: Karyotyping analysis can detect: Microscopic aberrations >5Mb
Chromosomes abnormalities:
- inversions
- duplications/deletions
- translocations
And please note that for cancer cells and in any cases where very complex, unstable karyotypes are observed.
- Q3: What species do you accept?
A: Specimens accepted include adherent and suspension cultures, stem cells, primary cells, and established cell lines. Cells on feeder layers or basement membranes are accepted. Human and mouse karyotyping comprise the majority of our work; please contact us regarding options for karyotyping other organisms and species. And please note that for cancer cells and in any cases where very complex, unstable karyotypes are observed.
For each species, there are three levels of service available:
- Cells growing in culture (which we process for analysis)
- Cells already processed and fixed in your lab (we provide a detailed protocol)
- Frozen cells (1-2 vials)
- Q4: What is the required information for sending my samples?
A: Please contact us to schedule prior to submission of cells for karyotyping analysis.
For each sample we require the following information (if available):
- Reasons for karyotyping analysis
- Cell type
- Species
- Gender (if known)
- Passage Number
- Q5: How long does it take to get karyotyping analysis results?
A: Turnaround time for cytogenetic testing varys, depending on numerous factors, such as the:
Type of testing requested (i.e., prenatal, cancer, etc.); Number of tests ordered per specimen; Complexity of results; and Quality of the sample submitted.
- Q6: What does a normal analysis constitute?
A: Since we are typically searching for abnormalities associated with cell culture, we report every cell with a structural abnormality. We would most likely not report cells with a single chromosome trisomy or monosomy as these could be found due to slide preparation (over-spreading). 10% mosaicism for structural or numerical abnormality would be excluded when practical in all instances. Cell lines with multiple cells with the same or different related abnormalities would be classed as cytogenetically abnormal. We would not assign the cell line as abnormal if an abnormality was found in a single cell only. Other species can be analyzed. Pricing and level of information that can be generated vary. Please contact us for further information.
- Q7: What are the content and form of the final results for the analysis?
A: The results will be sent as a pdf file by fax or by email (as requested). The sender will be supplied with an image of a metaphase chromosome spread and the karyotype from a cell obtained from the sample. To ensure confidentiality, results will not be provided by phone, unless the requesting agency wants it.
- Q8: What if I have questions about the test results?
A: Our genetic counselors are available to discuss the test results and can assist you in coordinating any additional testing you may need. Our goal is to provide a clear and comprehensive report that is understandable. However, we recognize that questions may arise, so our genetic counselors are available to discuss your questions or concerns.
Resources
Our Promise to You
Guaranteed product quality, expert customer support
