Drug-Drug Interaction
- Service Details
- Features
- FAQ
- Explore Other Options
Creative Bioarray providing drug metabolism and drug-drug interaction (DDI) study solutions to global pharmaceutical and biotech clients. Our services cover enzyme-mediated DDI (e.g., CYP450 inhibition/induction), transporter-mediated interactions, reaction phenotyping, and non-CYP metabolism. By leveraging advanced research methodologies and data analysis, we deliver reliable results that enhance drug development processes and mitigate associated risks.
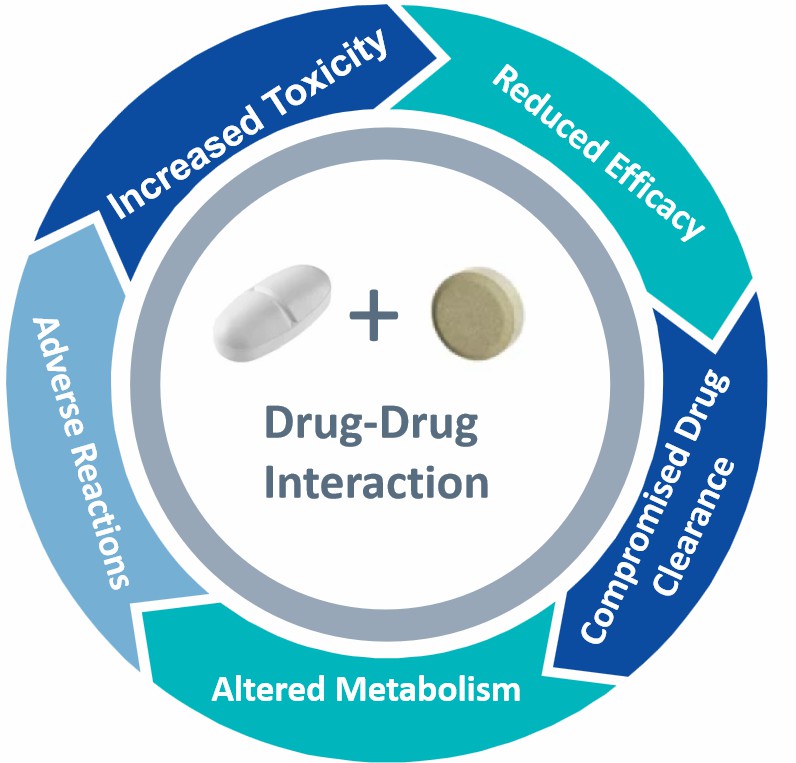
Why Test Drug-Drug Interaction?
Drug-drug interactions (DDI) form a critical part of drug development and clinical application because they can trigger harmful effects which may escalate to severe or fatal conditions. The simultaneous increase in elderly populations and extensive polypharmacy across the world points towards a future where drug-drug interactions will become more prevalent. Enzymes like cytochrome P450 (CYP450) and drug transporters play key roles in DDIs by altering drug metabolism, thereby affecting plasma and tissue drug levels. This modulation can impact therapeutic efficacy and safety. For instance, inhibition of CYP450 enzymes can elevate plasma drug levels, leading to toxicity, while enzyme induction can accelerate drug metabolism, reducing effectiveness or generating harmful metabolites.
Therefore, a thorough understanding of drug metabolic pathways, identification of key metabolic enzymes and transporters, and evaluation of potential drug interactions are essential for optimizing dosing regimens, minimizing medication risks, and ensuring patient safety. Creative Bioarray provides comprehensive drug metabolism and DDI studies to help clients address these challenges.
Our DDI Services
Cytochrome P450 Induction Assay
Designed to assess the capability of a test compound to induce cytochrome P450 (CYP450) enzymes, this assay focuses on how enzyme induction can enhance the metabolism of concurrently administered medications, potentially diminishing their therapeutic efficacy.
Cytochrome P450 Inhibition Assay
This assay evaluates the inhibitory effects of a test compound on the activity of cytochrome P450 (CYP450) enzymes. When inhibition occurs, it results in decreased drug metabolism which can elevate plasma drug concentrations and enhance the risk of toxic effects. Identifying such interactions is essential for ensuring drug safety and efficacy.
CYP450 Time Dependent Inhibition (TDI) Assay
Time dependent inhibition (TDI) refers to the phenomenon where certain compounds, after pre-incubation with CYP450 enzymes, gradually exhibit enhanced inhibitory effects on the enzymes over time. This inhibition is typically irreversible and may involve the formation of covalent bonds. Long-term medication safety evaluations require understanding time-dependent inhibition because it extends drug interactions and changes pharmacokinetics.
CYP and UGT Reaction Phenotyping Assay
CYP and UGT Reaction Phenotyping Assay is an experimental method used to identify specific cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT) enzyme subtypes involved in drug metabolism. This assay is vital for predicting potential drug-drug interactions and optimizing drug development strategies by clarifying the metabolic pathways involved.
UGT Induction
Focusing on the potential of a test compound to induce uridine diphosphate glucuronosyltransferases (UGTs), this assay shows how enzyme induction can lead to increased metabolism and clearance of co-administered drugs, which may reduce their efficacy.
UGT Inhibition (and Other Non-CYP Enzymes)
This assay evaluates the inhibitory effects of a test compound on the activity of uridine diphosphate glucuronosyltransferases (UGTs) and Other Non-CYP Enzymes. Inhibition of these enzymes can lead to reduced metabolism and increased plasma levels of co-administered drugs, potentially increasing the risk of toxicity.
P-gp and BCRP Inhibition Assay
We offer sensitive P-gp and BCRP inhibition assays to characterize efflux liability and its impact on drug absorption, brain penetration, and systemic exposure. These data help guide candidate selection and de-risk clinical development.
SLC Transporter Inhibition Assay
Using validated cell-based platforms, our assay quantifies inhibitory effects on major SLC transporters to clarify mechanistic contributions to ADME. These insights help guide pharmacokinetic optimization and early candidate selection.
Features

Highly Tailored Solutions
Provide comprehensive DDI services ranging from preliminary screening to in-depth mechanism studies according to client requirements.

Extensive Testing Panels
Our broad detection scope covers all aspects of drug-drug interactions.

Expert Team
Composed of experienced scientists and researchers who deliver professional consultation and technical support.
Rapid Turnaround
Optimized experimental workflows to shorten project timelines, and accelerate clients' drug development progress.

Stringent Quality Control
Adherence to international standards and industry guidelines ensures the highest quality across at all stage of the research.
FAQ
1. What contents are typically studied in DDI?
- Metabolism enzyme-mediated interactions (e.g., CYP450 inhibition or induction).
- Transporter-mediated interactions (e.g., P-glycoprotein's impact on drug absorption/distribution).
- Pharmacodynamic interactions (synergistic/antagonistic effects on drug targets or physiological pathways).
- Non-CYP metabolic pathways (e.g., UGT enzymes or esterase-mediated metabolism).
2. What is time-dependent inhibition (TDI)? Why is it more concerning than direct inhibition?
Time-dependent inhibition (TDI) refers to a compound's gradual (potentially irreversible) enhancement of enzyme inhibition over time after binding, rather than immediate inhibition. This delayed effect may lead to cumulative toxicity or long-term interaction risks, requiring critical evaluation in chronic disease treatments.
3. How do reaction phenotyping and metabolic stability studies differ?
Reaction phenotyping focuses on identifying enzymes involved in drug metabolism (e.g., CYP450/UGT isoforms), while metabolic stability studies assess the degradation rate of drugs in enzymatic systems. Together, they comprehensively characterize drug metabolism profiles.
4. If a drug is metabolized by both CYP450 and UGT, how to distinguish their contributions?
Use selective inhibitors (e.g., ketoconazole for CYP3A4) or gene-knockout cell lines to block specific enzyme activity, combined with mass spectrometry to quantify metabolite production and analyze the metabolic contribution of each enzyme.
5. Do you support combined testing (e.g., simultaneous TDI and reaction phenotyping)?
Yes! Combined testing saves sample volume and time costs. We offer discounted package pricing and ensure no interference between experimental modules.
Explore Other Options