Redefining Cell Therapy: The Direct-to-Patient Revolution
In vivo CAR-T therapy is redefining the future of cell immunotherapy. Rather than engineering T cells outside the body, this breakthrough approach reprograms a patient's immune cells in vivo using viral or non-viral vectors. The field is advancing rapidly, with multiple candidates entering clinical development and early data showing highly promising therapeutic efficacy across oncology and emerging autoimmune applications. This momentum is driving strong industry interest and significant investment.
As an off-the-shelf modality, in vivo CAR-T has the potential to overcome major limitations of traditional ex vivo manufacturing-high cost, complex logistics, and lengthy production cycles. However, unlocking its full potential still depends on solving key challenges, including achieving highly specific T-cell targeting and minimizing systemic toxicity caused by off-target biodistribution.
 (Uscanga-Palomeque et al., 2023)
(Uscanga-Palomeque et al., 2023)
Integrated Preclinical Solutions for In Vivo CAR-T Therapy
Accelerate your journey from concept to IND with a unified preclinical partnership
- CAR Molecule Engineering
- Delivery System Development
- In Vitro Delivery & Efficacy Screening
- In Vivo Efficacy in Translational Models
- Pharmacokinetics (PK) & Biodistribution (BD)
- Safety Assessment
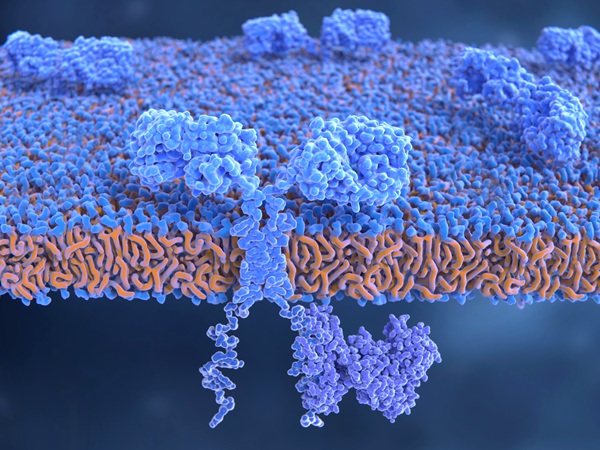
CAR Molecule Engineering
scFv/VHH Screening & Optimization
- Hybridoma Discovery
- Single B Cell Screening
- Human Naïve Library Screening
- Antibody Affinity Maturation
- sdAb Immunized Library Screening
- sdAb Naïve Library Screening
- Antibody Humanization
Structural Optimization
- Hinge Region Optimization
- Transmembrane Domain Optimization
- Intracellular Signaling Domain Optimization
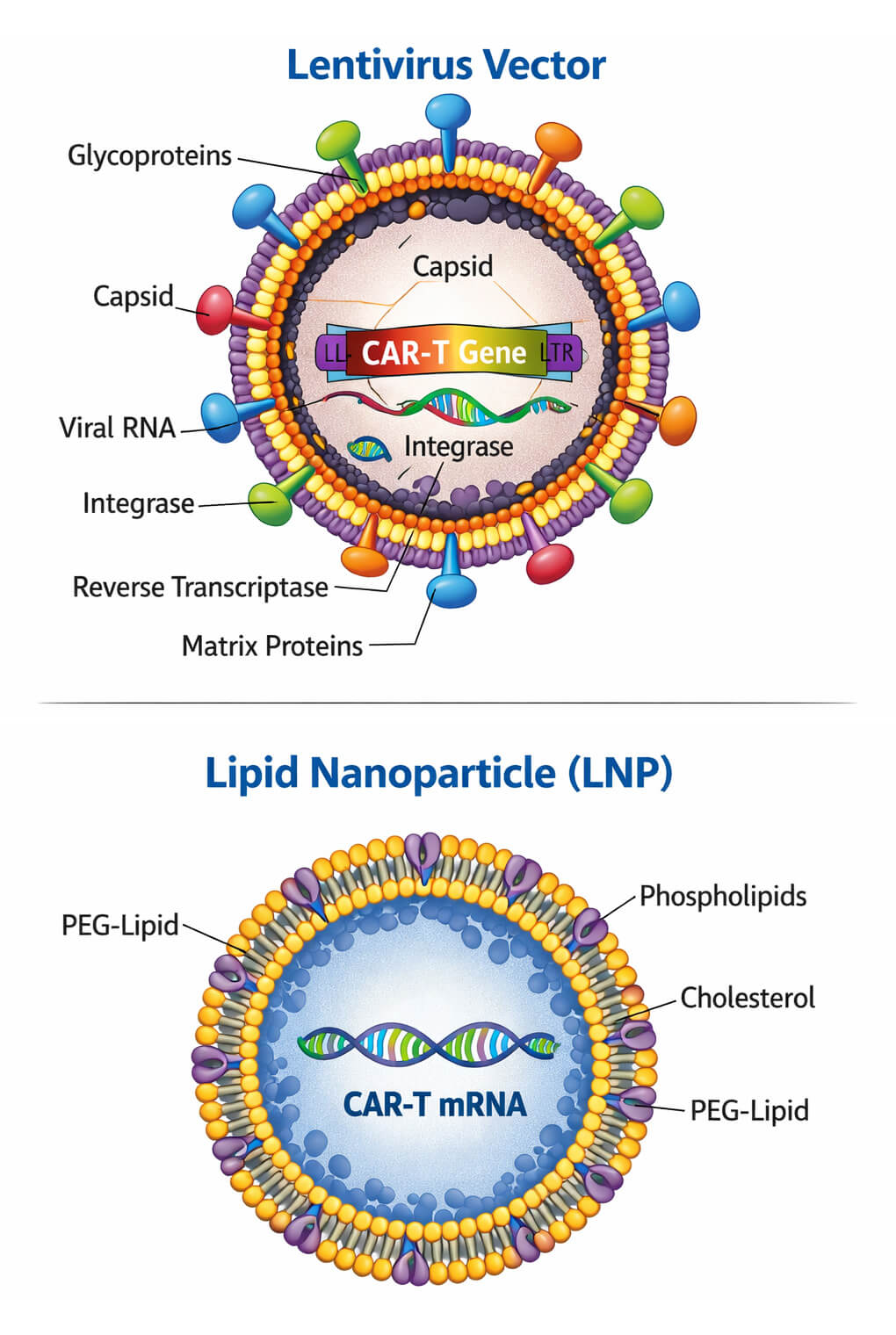
Delivery System Development
Viral Vector Engineering
Lentiviral Envelope Engineering
- VSV-G Replacement
- VSV-G Point Mutation
Lentiviral Tropism Refinement
Lentiviral Safety Engineering
- MHC Knockout
- CD47 Overexpression
Lentiviral Vector Structure Optimization
- Suicide Gene/Safety Switch
- Regulated Expression Module (RACP)
- T Cell-Specific Promoter
Non-Viral Delivery Engineering
Sequence Design & Vector Construction
- Plasmid Construction
- CAR mRNA Sequence Design & Optimization
LNP Formulation Screening
Targeting Optimization
- Antibody Design & Optimization
- Antibody-LNP Conjugation (Ab-LNP)

In Vitro Delivery & Efficacy Screening
- Validated Target Cell Model Establishment
- CAR Expression Kinetics & Stability
- Vector Tropism & Transduction Specificity
- Vector Screening & Cellular Compatibility
- Functional Efficacy Evaluation
In Vivo Efficacy in Translational Models
Pharmacokinetics (PK) & Biodistribution (BD)
- CAR-T Cell Pharmacokinetics (PK)
- CAR-T Cell Biodistribution (BD)
- CAR Expression Kinetics & Quantification
- Vector Pharmacokinetics (PK)
- Vector Biodistribution (BD)
Safety Assessment
Immunotoxicity
- Cytokine Release Syndrome (CRS) Assessment
- Immune Effector Cell-Associated Neurotoxicity (ICANS) Assessment
- Cytokine Profile Quantification
- Immune Cell Subset Analysis
Immunogenicity
Vector & Genetic Safety
- Genetic Integration Site Analysis
- Insertional Mutation Risk Assessment
- On-target/Off-target toxicity Assessment
General Toxicology

Why Partner with Us?
More than a CRO, we are your strategic R&D extension
End-to-End Preclinical Solutions
We provide fully integrated preclinical solutions for in vivo CAR-T therapies-from CAR design to vector engineering and in vitro/in vivo evaluation.

Custom Study Strategies
Our team designs flexible, tailored study strategies that adapt to evolving project needs, enabling rapid optimization and adaptive adjustments within our in vivo CAR‑T development platform.

Deep Therapeutic Expertise
Leverage our extensive experience in immuno-oncology and in vivo CAR-T therapy to navigate complex biology, optimize strategies, and advance programs efficiently.

Accelerated Translational Support
We streamline preclinical workflows to reduce turnaround time and support seamless progression from discovery to clinical translation.

Ready to Advance Your In Vivo CAR-T Program?
All inquiries receive a response from a senior scientist within 24 hours.
Talk to an Expert