Nitroglycerin (NTG)-Induced Migraine Model
Creative Bioarray has developed a range of migraine models, including the nitroglycerin (NTG)-induced migraine model, to evaluate the efficacy of innovative therapeutic agents. Our commitment to excellence ensures that our models are of the highest quality, offering unparalleled reliability and accuracy for your research needs. Contact us now to learn more about how our models can support your research objectives and help bring relief to migraine sufferers worldwide.
Migraine represents one of the most debilitating neurological conditions globally, posing significant challenges for study due to its diverse manifestations, episodic nature, and the intricate neural networks involved. However, both clinical and preclinical research has significantly advanced through the utilization of NTG, as a model for migraine. NTG is a highly permeable, lipophilic, organic nitrate that has been extensively used as a model of migraine. It is its action as a nitric oxide (NO) donor that is thought to mediate its migraine-inducing effects.
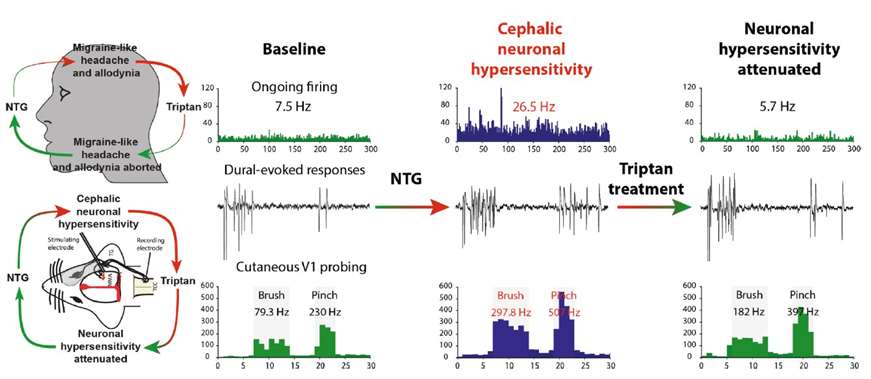 Fig. 1 Overview of the translational nature of nitroglycerin (NTG) as a model of migraine in experimental clinical and preclinical research. (Sureda-Gibert, P., et al, 2022)
Fig. 1 Overview of the translational nature of nitroglycerin (NTG) as a model of migraine in experimental clinical and preclinical research. (Sureda-Gibert, P., et al, 2022)
Our Nitroglycerin (NTG)-Induced Migraine Model
- Available Animal
- Rat
- Mouse
- Modeling Method
Animals are injected with NTG to induced migraine.
- Endpoints
- Clinical observation
- Behavioral tests
- 5-hydroxytryptamine (5-HT) and Nitric oxide (NO) analysis
- Histology analysis
- qPCR or Western blot
- Other customized endpoints
Example Data
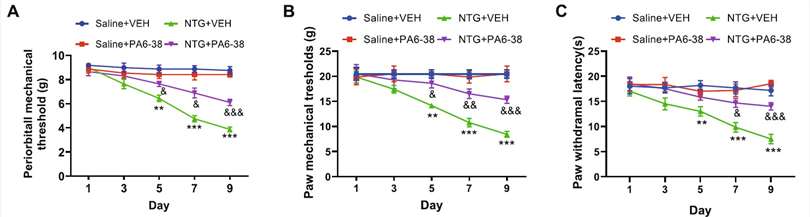 Fig. 2 PACAP6-38 treatment ameliorated the decreased mechanical (A-B) and thermal (C) thresholds after CM. (Zhang, L., et al, 2023)
Fig. 2 PACAP6-38 treatment ameliorated the decreased mechanical (A-B) and thermal (C) thresholds after CM. (Zhang, L., et al, 2023)
In addition, we also provide another migraine model that maybe you are interested in:
Quotation and Ordering
Creative Bioarray boasts a team of scientists with a demonstrated mastery of pharmacology and efficacy studies. We are committed to offering our clients comprehensive and personalized services of the highest quality at the most competitive prices. Our expertise enables us to tailor our approach to meet the specific needs of each project, ensuring optimal results and value for our clients. If you are interested in our services, please feel free to contact us at any time or submit an inquiry to us directly.
References
- Zhang, L., et al. PACAP6-38 improves nitroglycerin-induced central sensitization by modulating synaptic plasticity at the trigeminal nucleus caudalis in a male rat model of chronic migraine. J Headache Pain, 2023;24(1):66.
- Sureda-Gibert, P., et al. Nitroglycerin as a model of migraine: Clinical and preclinical review. Neurobiol Pain, 2022;12:100105.
For research use only. Not for any other purpose.
Disease Models
- Oncology Models
-
Inflammation & Autoimmune Disease Models
- Rheumatoid Arthritis Models
- Glomerulonephritis Models
- Multiple Sclerosis (MS) Models
- Ocular Inflammation Models
- Sjögren's Syndrome Model
- LPS-induced Acute Lung Injury Model
- Peritonitis Models
- Passive Cutaneous Anaphylaxis Model
- Delayed-Type Hypersensitivity (DTH) Models
- Inflammatory Bowel Disease Models
- Systemic Lupus Erythematosus Animal Models
- Oral Mucositis Model
- Asthma Model
- Sepsis Model
- Psoriasis Model
- Atopic Dermatitis (AD) Model
- Scleroderma Model
- Gouty Arthritis Model
- Carrageenan-Induced Air Pouch Synovitis Model
- Carrageenan-Induced Paw Edema Model
- Experimental Autoimmune Myasthenia Gravis (EAMG) Model
- Graft-versus-host Disease (GvHD) Models
-
Cardiovascular Disease Models
- Surgical Models
- Animal Models of Hypertension
- Venous Thrombosis Model
- Atherosclerosis model
- Cardiac Arrhythmia Model
- Hyperlipoidemia Model
- Doxorubicin-induced Heart Failure Model
- Isoproterenol-induced Heart Failure Model
- Arterial Thrombosis Model
- Pulmonary Arterial Hypertension (PAH) Models
- Heart Failure with Preserved Ejection Fraction (HFpEF) Model
- Cardio-Renal-Metabolic (CKM) Syndrome Model
-
Neurological Disease Models
- Alzheimer's Disease Modeling and Assays
- Seizure Models
- Parkinson's Disease Models
- Ischemic Stroke Models
- Acute Spinal Cord Injury (ASCI) Model
- Traumatic Brain Injury (TBI) Model
- Hypoxic-Ischemic Encephalopathy (HIE) Model
- Tourette Syndrome (TS) Model
- Amyotrophic Lateral Sclerosis (ALS) Model
- Huntington's Disease (HD) Model
- Intracerebral hemorrhage (ICH) Models
- Schizophrenia Model
- Depression Models
- Pain Models
-
Metabolic Disease Models
- Type 1 Diabetes Mellitus Model
- Type 2 Diabetes Mellitus Model
- Animal Model of Hyperuricemia
-
Nonalcoholic Fatty Liver Disease Model
- High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- Methionine and Choline Deficient (MCD) Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- Gubra-Amylin NASH (GAN) Diet-Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- Streptozotocin (STZ) Induced Nonalcoholic Fatty Liver Disease (NAFLD) Model
- High Fat Diet-Induced Obesity Model
- Diabetic Foot Ulcer (DFU) Model
- Cardio-Renal-Metabolic (CKM) Syndrome Model
- Liver Disease Models
- Rare Disease Models
- Respiratory Disease Models
- Digestive Disease Models
-
Urology Disease Models
- Cisplatin-induced Nephrotoxicity Model
- Unilateral Ureteral Obstruction Model
- 5/6 Nephrectomy Model
- Renal Ischemia-Reperfusion Injury (RIRI) Model
- Diabetic Nephropathy (DN) Models
- Passive Heymann Nephritis (PHN) Model
- Adenine-Induced Chronic Kidney Disease (CKD) Model
- Kidney Stone Model
- Doxorubicin-Induced Nephropathy Model
- Orthotopic Kidney Transplantation Model
- Benign Prostatic Hyperplasia (BPH) Model
- Peritoneal Fibrosis Model
- Cardio-Renal-Metabolic (CKM) Syndrome Model
- Orthopedic Disease Models
- Ocular Disease Models
- Skin Disease Models
- Infectious Disease Models
- Otology Disease Models