From Specimen to Slide: Core Methods in Histological Practice
Histology is the science of the microscopic architecture of normal human tissues and their functional relationships, focusing mainly on light and electron microscopy to see detailed architecture and functional connections, mostly in terms of morphological structure. Histology is widely used in medicine, biology and pathology. In biological research, histology investigates the normal and abnormal conditions of cells and tissues, providing vital morphological information for biomedical investigations.
Sample Collection and Processing
Biochemical samples include biomolecules, cells, tissues and organs, both healthy and diseased – human tissues, whole blood, plasma, serum, biological fluids, and processed samples (DNA, RNA, proteins). These specimens are often collected for the purpose of patient observation, medical treatment and basic, clinical and epidemiological studies. Many medical discoveries are grounded in studies of these specimens. Research samples are of varying origin and quality. It's the accuracy of the samples in the lab that makes a study successful. So sampling, processing, and storing of samples must be managed according to the standardized processes.
 Factors influencing the quality of biological samples can be grouped into two main categories that must be considered, whenever possible, when sampling and processing samples:
Factors influencing the quality of biological samples can be grouped into two main categories that must be considered, whenever possible, when sampling and processing samples:
| Pre-Analytic Factors | Analytic Factors |
| Physiological state of the sampled organism Uniformity in Biospecimen Collection Practices. Biospecimen Handling Procedures | Use of validated assays, where possible; Use of SOPs in which the technical staff are well-trained; Lot uniformity of reagents; Inclusion of appropriate type and number of quality control (reference) samples; Randomization, when possible |
It's important to choose the storage process for biological samples in accordance with the sample type, storage duration, and biomolecules to be studied. Biomaterials must remain in a stable condition, and unnecessary thawing and refreezing of frozen biological samples or biomolecules extracted from biological samples should be avoided.
Table. 1. General specimen storage guidelines (Vaught JB, Henderson MK, et al., 2011).
| Temperature in °C | Preservation method | Recommended for |
| +18 to +20 | Room temperature | Slides, tissue blocks |
| 0 to +4 | Refrigerator | Processing fresh specimens |
| -0.5 to-27 | Freezer | Short-term DNA stability |
| -27 to-40 | Freezer | DNA stability |
| -40 to-80 | Freezer | DNA/RNA stability |
| -80 to-130 | Freezer | Recommended for urine, blood, blood fractions (plasma, serum etc.) |
| -130 to-150 | Liquid nitrogen vapour | Recommended for storage of tissues, preservation of cellular viability |
| -196 | Liquid nitrogen liquid phase | Storage of living cells |
Sectioning
Specimen preparation techniques in histological studies are processes to convert tissue samples into forms suitable for microscopic observation, which can be categorized as sectioning and non-sectioning methods.
Sectioning methods
Paraffin sections
Paraffin sections are the most common form of sectioning, suitable for most tissue types, providing excellent structural details, long-term storage, and various staining methods. However, during section preparation, tissues are treated with organic solvents like alcohol and clearing agents, which can lead to significant loss of antigen activity within the tissues.
The process includes sampling, fixation, dehydration, clearing, paraffin infiltration, embedding, sectioning, and staining.
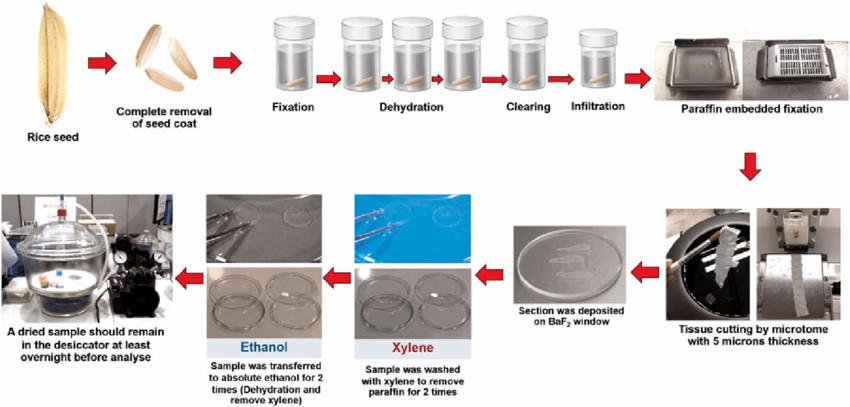 Fig. 1. paraffin embedding and tissue section process (Siriwong S, Tanthanuch W, et al., 2022)
Fig. 1. paraffin embedding and tissue section process (Siriwong S, Tanthanuch W, et al., 2022)
Frozen sections
Frozen sections are used in situations requiring rapid diagnosis, such as intraoperative rapid pathology. As the tissues do not undergo chemical fixation, they can better preserve enzyme activity and antigenicity, making them suitable for experiments like immunohistochemistry, enzyme histochemistry, and in situ hybridization where biological activity needs to be retained. Since frozen sections do not undergo conventional dehydration and clearing processes, they tend to be softer, prone to curling and breaking, typically about 5-10 μm thick, and not ideal for long-term preservation. The steps include tissue collection, freezing, sectioning, slide mounting, optional fixation and staining, and microscopic examination.
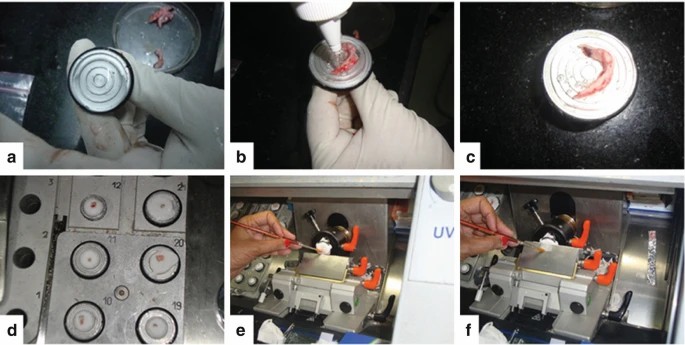 Fig. 2. Cryostat processing: (a) Mould is covered with OCT, (b) tissue is now put on the block, (c) OCT is flooded over the tissue, (d) Tissue now is put in the cooling chamber, (e) The brush guides the tip of the tissue, (f) The tissue section is gently spread over the antiroll plate and later picked up by touching a glass slide (Dey P, 2022).
Fig. 2. Cryostat processing: (a) Mould is covered with OCT, (b) tissue is now put on the block, (c) OCT is flooded over the tissue, (d) Tissue now is put in the cooling chamber, (e) The brush guides the tip of the tissue, (f) The tissue section is gently spread over the antiroll plate and later picked up by touching a glass slide (Dey P, 2022).
Celloidin sections
Celloidin sections use celloidin as an embedding medium for slicing large tissue blocks, like the eyeball or testes, retaining the tissue structure well. However, tissue shrinkage or deformation may occur during sectioning.
Non-sectioning methods
Non-sectioning techniques provide important methods for histological studies other than sectioning. These techniques process and observe tissues or cells in ways that allow obtaining information about the morphology, structure, and function of tissues and cells without sectioning.
Smear technique: Involves smearing liquid specimens (such as blood, semen, sputum, pleural fluid, ascitic fluid, etc.) or cell suspensions prepared after processing evenly on a slide for observation.
Spread technique: Mainly used to observe tissues with a certain level of structural organization, like mesentery, omentum, or nerve fibers. After spreading, one can proceed with fixation, staining, etc., as required. This technique visually displays the planar structure of tissues.
Squash technique: Entails placing fresh or fixed tissues on a slide, covering with a coverslip, and applying appropriate pressure to crush or spread the tissues, enabling cell dispersion for observation. Suitable for soft-textured tissues where cells disperse easily, such as root tips, anthers in plants, and embryos or soft tissues in animals.
Grinding technique: Primarily used for studying hard tissues like bones and teeth. The hard tissue specimens are cut and ground to make thin sections for viewing the internal structure under a microscope.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
| Sample Collection | Creative Bioarray provides pharmaceutical and biotech industry with sample collection and analysis services to researchers worldwide. Our capabilities include collecting patient samples, plasma, whole blood, serum, urine, saliva, tissue and other biological specimens on request. |
| Tissue Sections | Creative Bioarray offers paraffin embedded and frozen tissue sections. Tissues available include human adult and fetal normal tissues, human diseased and tumor tissues, as well as from mouse, rat, and monkey tissues. |
| FFPE Cell Pellet | Creative Bioarray offers the most comprehensive list of high-quality and affordable FFPE cell pellets to best fit your specific research objectives. |
References
- Siriwong S, Tanthanuch W, et al. Performance Evaluation of Focal Plane Array (FPA)-FTIR and Synchrotron Radiation (SR)-FTIR Microspectroscopy to Classify Rice Components. Microsc Microanal. 2022. 5:1-10.
- Dey, P. Frozen Section: Principle and Procedure. In: Basic and Advanced Laboratory Techniques in Histopathology and Cytology. Springer, Singapore. 2022