From Cells to Systems: Modern Approaches to Disease Modeling
We use disease models to understand the biological processes that drive disease. Disease models have the capacity to recapitulate the complex genetic, biochemical, physiological, and environmental factors that together play an important role in disease initiation, development, and resolution. As such, disease models are a cornerstone of biomedical research, and have been utilized throughout the history of biomedical research. Models of human disease provide an opportunity to expand the current understanding of disease mechanisms, therapeutic targets, and even drug efficacy. In the context of drug development, disease models have numerous roles including:
- Helping to understand disease etiology and signaling pathways
- Helping to identify and validate drug targets
- Helping to determine drug efficacy, toxicity, and pharmacodynamics
- Helping with biomarker discovery and patient stratification
- Helping to decrease late-stage clinical failures
While many disease models have been developed, we still do not have one that can completely recapitulate disease in humans. Selecting the most appropriate model for each disease stage, and integrating the results from different but complementary models is critical. We aim to help you select the most appropriate model that matches your mechanistic depth of interest and your translational needs in order to help you make the right decision in the drug development pipeline.
Types of Disease Models: Mechanisms and Applications
1. In Vitro Disease Models (Cell-Based Systems)
In vitro models are the simplest form of disease investigation, which allow for the greatest degree of experimental control and direct manipulation of cellular and molecular parameters in culture. These features make in vitro models the most widely used for mechanistic interrogation, high-throughput screening, and early-stage validation.
Types of In Vitro Models
- Primary cells derived from healthy or diseased tissues, preserving physiological relevance
- Immortalized cell lines, offering reproducibility and scalability
- Stem cell-derived models, including iPSC-derived differentiated cells
- 3D cultures and organoids, recapitulating tissue architecture and cell-cell interactions
- Co-culture systems, modeling interactions between multiple cell types (e.g., tumor-immune, neuron-glia)
Mechanistic Insights
Cell-based disease models allow for the direct interrogation of intracellular signaling cascades, gene expression, metabolic changes, and cellular phenotypes such as proliferation, apoptosis, inflammation, and differentiation. Genetic perturbation tools (e.g., CRISPR/Cas9, RNAi), pharmacological modulation, and omics-based readouts can further enhance mechanistic resolution.
Applications
- Target identification and validation
- Mechanism-of-action (MoA) studies
- Phenotypic and pathway-based drug screening
- Toxicity and safety profiling
- Biomarker exploration
While in vitro models offer convenience, efficiency, and experimental control, they are often limited in systemic relevance and may not recapitulate the disease state of interest. Therefore, their predictive value is most enhanced when combined with higher-order disease models.
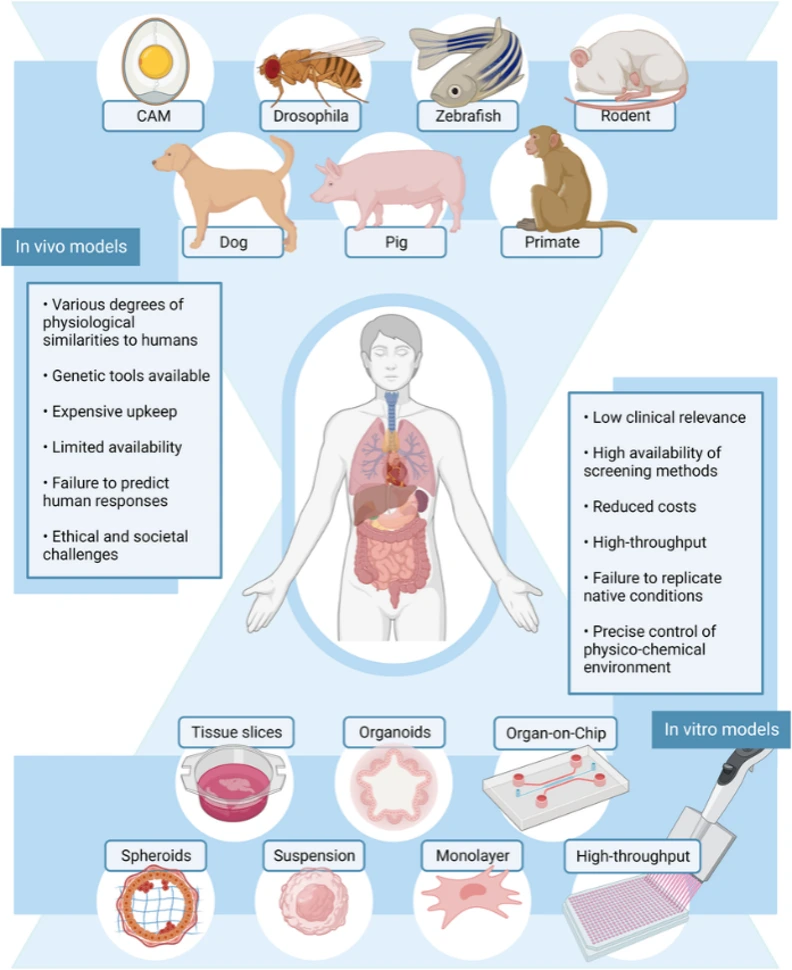
2. In Vivo Disease Models (Animal Models)
In vivo models, or animal models, represent a step up in complexity and provide the necessary systemic context to capture the interplay of tissues, organs, immune responses, metabolism, and drug disposition. Animal models are the most established and broadly applied disease models in preclinical research and are often used to study disease progression and test therapeutic interventions.
Common Animal Models
- Rodent models (mouse and rat), including transgenic, knockout, and knock-in strains
- Disease induction models, such as chemical, surgical, or infectious induction
- Humanized models, incorporating human genes, cells, or immune systems
- Large animal models, used when closer physiological similarity is required
Mechanisms Explored
Animal models allow for the exploration of disease mechanisms at the tissue and whole-organism level, such as inflammation, fibrosis, neurodegeneration, tumor growth, immune dysregulation, metabolic dysfunction, and organ pathology. Longitudinal disease progression and therapeutic intervention can also be assessed.
Applications
- Proof-of-concept and efficacy studies
- Dose optimization and pharmacodynamics
- Safety, tolerability, and target engagement
- Biomarker validation in physiological context
- Regulatory-supporting preclinical data generation
Despite their value, animal models often fail to fully predict human clinical outcomes due to species-specific differences. Careful model selection, validation, and ethical consideration are therefore critical.
3. Computational and In Silico Disease Models
Computational models refer to a rapidly growing class of disease models that leverage mathematical modeling, bioinformatics, and artificial intelligence (AI) to simulate disease processes and predict outcomes.
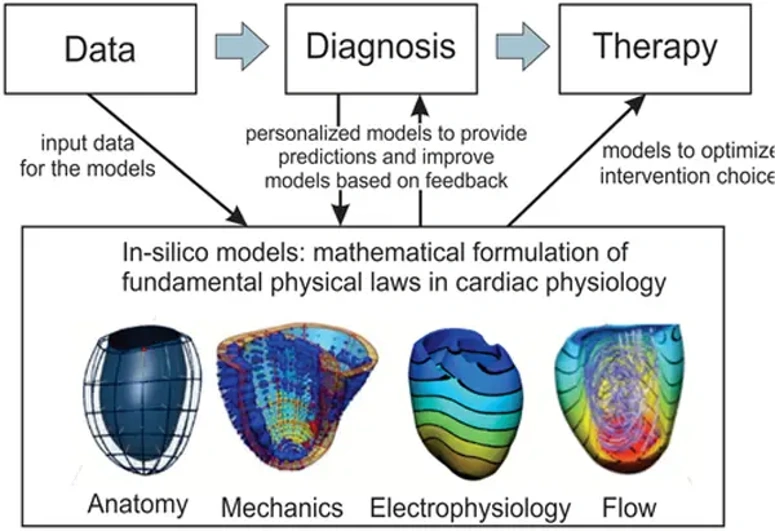
Types of Computational Models
- Systems biology models, describing molecular networks and pathway dynamics
- PK/PD models, linking drug exposure to biological response
- Population and disease progression models, capturing variability across individuals
- Machine learning-based models, integrating multi-omics and clinical data
Mechanistic Value
Computational models offer the ability to generate hypotheses, identify key regulatory nodes and mechanisms, and predict system-level behavior that is difficult, expensive, or time-consuming to measure experimentally. This is especially true for models that are trained on large and complex datasets and can be used to explore "what-if" scenarios in silico.
Applications
- Target prioritization and network analysis
- Virtual screening and drug optimization
- Dose and regimen prediction
- Biomarker and patient stratification modeling
- Reduction of experimental burden and animal use
While computational models depend heavily on data quality and assumptions, their integration with experimental models significantly enhances efficiency and insight.
Challenges Facing Disease Modeling
While disease modeling has made significant progress, several limitations and challenges still need to be addressed.
Species Differences
One of the main challenges of disease modeling is the biological gap between animal models and humans, which can limit the translational value of preclinical findings. Mouse models have been widely used in biomedical research, but they often fail to recapitulate the full complexity of human diseases, particularly those with genetic and environmental heterogeneity. This has prompted the development of more human-relevant models, such as humanized animal models and organ-on-a-chip systems, to enhance the predictive power of preclinical studies.
Model Complexity
Human diseases are often complex and involve interactions between multiple cell types, tissues, and organs. This complexity can be difficult to capture in a single disease model. In vitro systems can provide mechanistic clarity but lack systemic context, while in vivo models offer physiological relevance at the cost of increased complexity, time, and ethical considerations. The challenge is to find an optimal balance between biological relevance, experimental feasibility, and ethical responsibility.
Data Integration and Interpretation
The recent advances in genomics, proteomics, and imaging technologies have generated large and complex datasets in disease research. While these data can provide valuable insights into disease mechanisms, their integration and interpretation require advanced computational tools and cross-disciplinary expertise. Data quality, reproducibility, and consistency across platforms are critical for building reliable and translatable disease models.
Ethical and Regulatory Considerations
Animal models are often associated with ethical and regulatory issues related to animal welfare. While efforts have been made to refine experimental approaches and develop alternative models, these issues remain a challenge. Navigating ethical expectations and regulatory requirements is an ongoing process for researchers and companies involved in disease model development.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
|---|---|
| Disease Models | Our comprehensive portfolio of disease models spans multiple therapeutic areas, providing reliable preclinical platforms to study disease mechanisms and accelerate the development of innovative therapies. |
References
- Yildirim Z, Swanson K, et al. Next-Gen Therapeutics: Pioneering Drug Discovery with iPSCs, Genomics, AI, and Clinical Trials in a Dish. Annu Rev Pharmacol Toxicol. 2025 Jan;65(1):71-90.
- Panayides AS, Amini A, et al. AI in Medical Imaging Informatics: Current Challenges and Future Directions. IEEE J Biomed Health Inform. 2020, 24(7):1837-1857.