Melanoma Cells
- Background
- Applications
- Scientific Data
- FAQ
Melanoma, a type of skin cancer, arises from the pigment-producing cells known as melanocytes. Melanocytes are responsible for producing the pigment melanin, which protects the skin from ultraviolet (UV) radiation. When these cells undergo genetic mutations, they can become malignant, leading to melanoma. Melanoma cells are characterized by their ability to proliferate rapidly and invade surrounding tissues, contributing to the disease's aggressive nature.
Melanoma Cell Growth and Proliferation
Melanoma cells possess a remarkable capacity to circumvent the normal cellular checks and balances that govern cell growth and division. Through a complex interplay of genetic mutations and dysregulated signaling pathways, these cells can evade the natural mechanisms that typically limit cell proliferation.
- The key driver of melanoma cell proliferation is the activation of the Mitogen-activated protein kinase (MAPK) signaling cascade. This critical pathway, which is responsible for transmitting growth signals from the cell surface to the nucleus, is frequently hyperactivated in melanoma cells due to mutations in genes such as BRAF and NRAS. These genetic alterations lead to the sustained activation of the MAPK pathway, promoting the unchecked division and rapid expansion of the malignant cells.
- Some researchers have identified the crucial role of cell cycle regulators, such as the cyclin-dependent kinase inhibitor p16INK4A, in the uncontrolled growth of melanoma cells. Mutations or epigenetic silencing of these cell cycle checkpoints allow melanoma cells to bypass the normal constraints on cell division, fueling their relentless proliferation.
Melanoma Cell Invasion and Metastasis
The hallmark of melanoma's lethality lies in its ability to invade surrounding tissues and spread to distant organs through a process known as metastasis.
- Factors driving melanoma cell invasion is the dysregulation of cell-cell adhesion molecules, such as E-cadherin and N-cadherin. Melanoma cells often exhibit a "cadherin switch," where the expression of E-cadherin, which typically maintains cell-cell adhesion, is downregulated, while the expression of N-cadherin, which promotes cell-matrix interactions, is upregulated. This cadherin switch allows the melanoma cells to detach from their neighbors and engage with the surrounding extracellular matrix, facilitating their migratory potential.
- Matrix metalloproteinases (MMPs) also play roles in the invasion and metastasis of melanoma cells. These enzyme complexes are capable of degrading the extracellular matrix, creating pathways for the malignant cells to navigate through the surrounding tissues. Elevated levels of MMPs, such as MMP-2 and MMP-9, have been consistently observed in metastatic melanoma, highlighting their importance in the dissemination of this deadly cancer.
Cancer Biology and Pathogenesis
Melanoma cells serve as a model to study the fundamental processes of cancer biology, including apoptosis, autophagy, and cell signaling pathways. This research provides insights into the pathogenesis of melanoma and other cancers, leading to the identification of new therapeutic targets.
Predictive and Prognostic Biomarkers
Melanoma cells are instrumental in identifying biomarkers that can predict patient outcomes and response to therapy. For instance, the presence of specific mutations in genes like BRAF or NRAS can indicate a higher likelihood of response to targeted therapies.
Drug Resistance and Combination Therapies
The development of resistance to single-agent therapies is a significant challenge in melanoma treatment. Melanoma cells are used to study the mechanisms of drug resistance and to test combination therapies that can overcome or prevent it. By understanding how melanoma cells evolve resistance, researchers can design more effective treatment strategies.
Tumor Heterogeneity and Clonal Evolution
Tumor heterogeneity is a hallmark of cancer, and melanoma is no exception. Melanoma cells are used to investigate the clonal evolution of tumors, which is the process by which different populations of cancer cells arise and evolve within a single tumor. Researchers use advanced sequencing technologies to analyze the genetic diversity within melanoma cells, which is crucial for understanding tumor progression and treatment resistance.
Adipocytes Increase Melanoma Cell Proliferation and Invasion
Melanomas arise at the dermal-epidermal junction, where they initially expand in the radial growth phase. During progression, these lesions extend down into the dermal tissue during the vertical growth phase, and some lesions continue to grow into the subcutaneous tissues, where they are then classified as Clark's level V. Moreover, metastatic tumor cells can also reach subcutaneous tissues as in-transit metastases. Histologic examination of these advanced melanomas demonstrates that these tumors are encased by subcutaneous adipocytes present in the tumor microenvironment (Fig. 1A). Adipocytes directly adjacent to the tumor are diminished in size compared to those further away, consistent with tumor-induced lipolysis.
To assess the role of adipocytes in melanoma progression, an adipocyte-melanoma cell co-culture system was established (Fig.1B). 3T3-L1 cells were differentiated into adipocytes with mature lipid droplets and then grew human A375 or SKMel28 cells on top. The proliferation of melanoma cells with or without the adipocytes was measured using pH3 staining (Fig. 1C). In both cell lines, a significant increase in melanoma cell growth when the cells were serum starved to remove other growth factors.
Whether the adipocytes could also increase melanoma cell invasion was tested using several different assays. Adipocyte-conditioned media increased the ability of A375 melanoma cells to degrade a gelatin matrix in 2D (Fig. 1D). Transwell assays were utilized to measure if this increased the ability of the cells to invade through the membrane. Melanoma cells were treated with adipocyte-conditioned media and found that this significantly increased the ability of the cells to invade into a collagen plug contained within the Transwell (Fig. 1E). Finally, melanoma cells were co-cultured with or without adipocytes, FACS-isolated the melanoma cells, and found that the adipocyte-exposed melanoma cells had a significantly increased ability to fully traverse a Transwell membrane that had been coated with Matrigel (Fig. 1F). Collectively, these data indicate that adipocytes can increase melanoma cell aggressiveness.
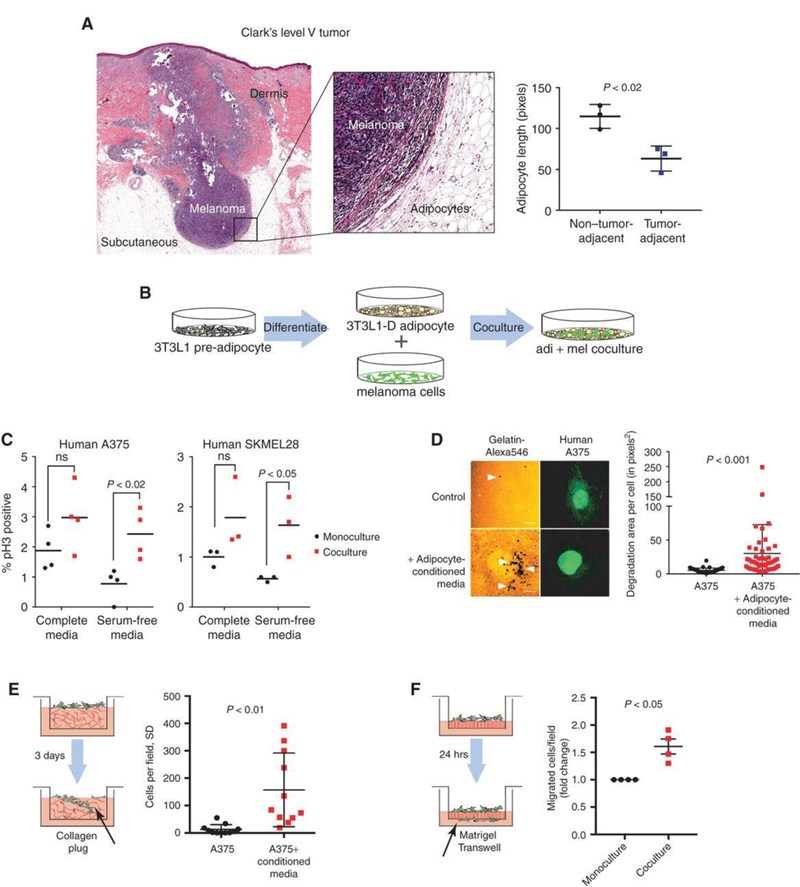 Fig.
1 Tumor-adjacent adipocytes contribute to melanoma progression. (Zhang M, et al, 2018)
Fig.
1 Tumor-adjacent adipocytes contribute to melanoma progression. (Zhang M, et al, 2018)
Exosomal Proteins for Diagnosis in Melanoma Subjects
To determine the significance of circulating exosome levels in metastatic melanoma, exosomes were prospectively isolated and characterized from the plasma of human subjects with increasing clinical stages. Exosomes (100 nm particles) were isolated using standard methods and confirmed their identity by electron microscopy (Fig. 2a). Known exosome markers including CD63, CD9, and MHC-I were identified (data not shown). Exosome size distribution and number, quantified by NanoSight analysis, did not differ based on clinical stage. In contrast, exosome protein concentrations were higher in subjects with Stage IV disease compared to all other stages and normal controls. Furthermore, Stage IV individuals with protein-poor exosomes (< 50 μg ml-1) had a survival advantage compared to those with protein-rich exosomes (> 50 μg ml-1) (Fig. 2b).
Exosomes from highly metastatic mouse (B16-F10) and human (SK-Mel28/-202/-265/-35) melanoma cell lines expressed typical exosome markers. Mass spectrometry led to the identification of additional human and mouse-derived melanoma exosome proteins that helped define a potential diagnostic "signature" in melanoma-derived exosomes. TYRP2 (a melanoma-specific protein), VLA-4, and HSP70 levels were increased in the exosomes of subjects with Stage IV disease (Fig. 2c, d) while only TYRP2 was significantly increased (P < 0.05) in Stage III individuals, as compared to control subjects (Fig. 2c, d). Interestingly, an isoform of HSP90, reported to be important in cellular transformation, was found in 70% of Stage IV melanoma exosomes (Fig. 2c). Exosomal TYRP2 levels were significantly increased (P < 0.001) in exosomes correlated with metastatic progression in a retrospective cohort of individuals with Stage III melanoma (Fig. 2e). These data support the use of analyzing circulating exosomes to predict prognosis (Stage III) and outcome (Stage IV) for subjects with melanoma.
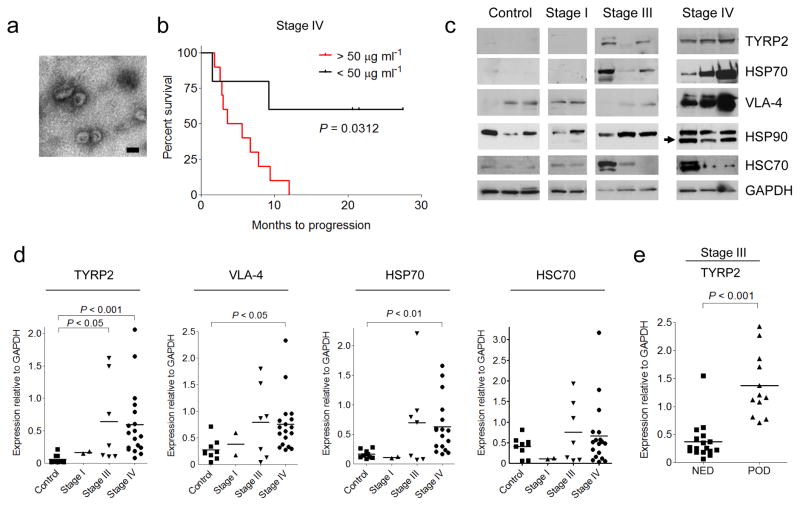 Fig.
2 Analysis of protein expression in circulating exosomes from melanoma subjects. (Peinado H, et al.,
2016)
Fig.
2 Analysis of protein expression in circulating exosomes from melanoma subjects. (Peinado H, et al.,
2016)
The growth and proliferation of melanoma cells are influenced by several factors, including genetic mutations (such as BRAF and NRAS), dysregulation of the cell cycle, and the activation of growth factor signaling pathways. Additionally, interactions with the tumor microenvironment, such as angiogenesis and immune response, can also contribute to tumor growth.
Melanoma cells invade surrounding tissues by secreting enzymes that break down the extracellular matrix, allowing them to penetrate blood vessels. This process, known as invasion, is followed by metastasis, where cancer cells travel through the bloodstream to distant organs. The ability of melanoma cells to undergo epithelial-mesenchymal transition (EMT) and to evade the immune system are critical steps in the metastatic process.
Research on melanoma cells contributes significantly to our understanding of cancer biology by providing insights into the molecular and cellular mechanisms that drive tumor initiation, progression, and metastasis. It also aids in the discovery of new therapeutic targets and the development of models that accurately represent human disease, thereby accelerating the translation of research findings into clinical practice.
Filters Clear all filters
Species
- African clawed frog (1)
- American mink (1)
- Asian tiger mosquito (1)
- Atlantic salmon (1)
- Bluegill (2)
- Bluestriped grunt (1)
- Bovine (7)
- Brazilian free-tailed bat (1)
- Brown bullhead (2)
- Cabbage looper (1)
- Cabbage moth (6)
- Cat (4)
- Central mudminnow (1)
- Chicken (3)
- Chinese hamster (5)
- Chinook salmon (2)
- Chum salmon (1)
- Coho salmon (1)
- Common carp (2)
- Cotton-top tamarin (1)
- Dog (2)
- Fall armyworm (3)
- Fathead minnow (2)
- Fruit fly (1)
- Gilthead sea bream (2)
- Golden hamster (7)
- Goldfish (6)
- Gray dwarf hamster (1)
- Green monkey (2)
- Gypsy moth (1)
- Horse (1)
- Human (998)
- Japanese eel (1)
- Japanese rice fish (7)
- Koi carp (1)
- Mouse (315)
- Mouse x Gray dwarf hamster (1)
- Mouse x Rat (20)
- Northern pike (1)
- Pig (3)
- Rabbit (2)
- Rainbow trout (3)
- Rat (115)
- Rhesus macaque (1)
- Salt marsh moth (1)
- Sheep (2)
- Snakehead murrel (2)
- Sockeye salmon (1)
- Vervet monkey (2)
- Zebrafish (2)
Source
- Abdomen (1)
- Abdomen Metastasis (2)
- Adipose (2)
- Adrenal Gland (8)
- Adrenal Gland Metastasis (2)
- Aorta (4)
- Artery (1)
- Ascites (28)
- Ascites Metastasis (37)
- Bile Duct (3)
- Bladder (25)
- Bladder Metastasis (1)
- Blastocyst (1)
- Blastula (1)
- Blood (127)
- Bone (27)
- Bone Marrow (57)
- Bone Marrow Metastasis (18)
- Bone Metastasis (6)
- Brain (55)
- Brain Metastasis (8)
- Breast (30)
- Bronchus (1)
- Caudal Peduncle (1)
- Caudal Trunk (2)
- Cecum (3)
- Cerebrospinal Fluid (1)
- Cerebrospinal Fluid Metastasis (1)
- Cervix (32)
- Colon (90)
- Connective Tissue (7)
- Cornea (3)
- Cutaneous Metastasis (1)
- Dermis (2)
- Duodenum (1)
- Embryo (29)
- Endometrium (17)
- Esophagus (44)
- Eye (12)
- Eye Socket (5)
- Fetus (3)
- Fin (9)
- Foreskin (4)
- Gallbladder (1)
- Gingiva (2)
- Globe (2)
- Glomerulus (2)
- Groin (1)
- Head Kidney (2)
- Heart (4)
- Hemolymph (1)
- Hypodermis Metastasis (5)
- Ileum (1)
- Intestine (94)
- Jejunum (1)
- kidney (1)
- Kidney (27)
- Liver (35)
- Liver Metastasis (17)
- Lung (58)
- Lung Metastasis (8)
- Lymph Node (7)
- Lymph Node Metastasis (59)
- Muscle (7)
- Muscle Metastasis (2)
- Nose (2)
- Omentum Metastasis (2)
- Oral Cavity (10)
- Ovary (21)
- Ovary Metastasis (2)
- Pancreas (19)
- Pelvic Wall Metastasis (1)
- Pelvis (1)
- Perianal Space Metastasis (1)
- Pericardial Effusion (1)
- Pericardial Effusion Metastasis (2)
- Perineus (1)
- Peripheral Blood (126)
- Peripheral Nervous System (21)
- Peritoneal Effusion (2)
- Peritoneum (1)
- Peritoneum Metastasis (1)
- Pharynx (3)
- Pituitary Gland (7)
- Pleural Effusion (54)
- Pleural Effusion Metastasis (46)
- Prostate (7)
- Rectum (15)
- Renal Pelvis (1)
- Retroperitoneal Space (2)
- Salivary Gland (2)
- Skeletal Muscle (5)
- Skin (32)
- Skin Metastasis (3)
- Small Intestine (4)
- Small Intestine Metastasis (1)
- Smooth Muscle (2)
- Soft Tissue (1)
- Soft Tissue Metastasis (1)
- Spinal Cord (2)
- Stomach (4)
- Testis (15)
- Thoracic Cavity Metastasis (6)
- Thymus (5)
- Thyroid Gland (16)
- Thyroid Gland Metastasis (1)
- Tongue (5)
- Trachea (1)
- Umbilical Cord (1)
- Umbilical Cord Blood (1)
- Urachus (1)
- Ureter (1)
- Uterus (54)
- Uvea (2)
- Vagina (2)
- Vulva (1)
Disease
- Acute Biphenotypic Leukemia (1)
- Acute Erythroid Leukemia (4)
- Acute Megakaryoblastic Leukemia (4)
- Acute Monocytic Leukemia (9)
- Acute Myeloid Leukemia (25)
- Acute Promyelocytic Leukemia (2)
- Adrenal Gland Neuroblastoma (11)
- Adult B Acute Lymphoblastic leukemia (1)
- Adult B Acute Lymphoblastic Leukemia (6)
- Adult T Acute Lymphoblastic Leukemia (6)
- Adult T Lymphoblastic Lymphoma (2)
- Adult T-Cell Leukemia/Lymphoma (1)
- Alveolar Rhabdomyosarcoma (4)
- Alveolar Ridge Squamous Cell Carcinoma (1)
- Amelanotic Melanoma (3)
- Ampulla of Vater Adenocarcinoma (1)
- Ampulla of Vater Adenosquamous Carcinoma (3)
- Anaplastic Astrocytoma (3)
- Anaplastic Large Cell Lymphoma (7)
- Askin Tumor (1)
- Astrocytoma (5)
- B Acute Lymphoblastic Leukemia (2)
- B-Cell Non-Hodgkin Lymphoma (5)
- Bare Lymphocyte Syndrome Type 2 (1)
- Barrett Adenocarcinoma (2)
- Benign Prostatic Hyperplasia (1)
- Bladder Carcinoma (14)
- Bladder Squamous Cell Carcinoma (1)
- Bovine Leukemia (2)
- Breast Adenocarcinoma (4)
- Breast Carcinoma (9)
- Breast Ductal Carcinoma (2)
- Burkitt Lymphoma (17)
- Canavan Disease (1)
- Canine Histiocytic Sarcoma (1)
- Cecum Adenocarcinoma (3)
- Central Nervous System Lymphoma (2)
- Cervical Adenocarcinoma (2)
- Cervical Adenosquamous Carcinoma (2)
- Cervical Small Cell Carcinoma (1)
- Cervical Squamous Cell Carcinoma (2)
- Chicken Bursal Lymphoma (2)
- Childhood B Acute Lymphoblastic Leukemia (13)
- Childhood T Acute Lymphoblastic Leukemia (16)
- Childhood T Lymphoblastic Lymphoma (1)
- Cholangiocarcinoma (2)
- Chronic Eosinophilic Leukemia (1)
- Chronic Lymphocytic Leukemia (2)
- Chronic Myeloid Leukemia (23)
- Clear Cell Renal Cell Carcinoma (2)
- Colon Adenocarcinoma (55)
- Colon Carcinoma (34)
- Colorectal Adenocarcinoma (1)
- Colorectal Carcinoma (1)
- Congenital Pure Red Cell Aplasia (1)
- Cutaneous Melanoma (10)
- Dedifferentiated Chondrosarcoma (1)
- Desmoplastic Melanoma (1)
- Diffuse Large B-Cell Lymphoma (28)
- Down Syndrome (2)
- EBV-Related Burkitt Lymphoma (12)
- Embryonal Carcinoma (3)
- Embryonal Rhabdomyosarcoma (3)
- Endometrial Adenocarcinoma (13)
- Endometrial Adenosquamous Carcinoma (2)
- Endometrial Carcinoma (2)
- Endometrioid Stromal Sarcoma (1)
- Epithelioid Hemangioendothelioma (1)
- Epithelioid Sarcoma (3)
- Esophageal Adenocarcinoma (6)
- Esophageal Squamous Cell Carcinoma (41)
- Essential Thrombocythemia (1)
- Ewing Sarcoma (2)
- Extraskeletal Myxoid Chondrosarcoma (1)
- Fanconi Anemia (1)
- Fibrosarcoma (1)
- Follicular Lymphoma (2)
- Gallbladder Carcinoma (2)
- Gallbladder Undifferentiated Carcinoma (2)
- Gastric Adenocarcinoma (6)
- Gastric Adenosquamous Carcinoma (1)
- Gastric Carcinoma (5)
- Gastric Choriocarcinoma (1)
- Gastric Fundus Carcinoma (1)
- Gastric Signet Ring Cell Adenocarcinoma (1)
- Gastric Small Cell Carcinoma (2)
- Gastric Tubular Adenocarcinoma (5)
- Gastroesophageal Junction Adenocarcinoma (1)
- Gestational Choriocarcinoma (1)
- Gingival Squamous Cell Carcinoma (2)
- Glioblastoma (18)
- Gliosarcoma (1)
- Goldfish Erythrophoroma (4)
- Hairy Cell Leukemia (1)
- Hamster Kidney Tumor (1)
- Hamster Pancreatic Ductal Adenocarcinoma (1)
- Hamster Uterine Leiomyosarcoma (1)
- Hepatoblastoma (2)
- Hepatocellular Carcinoma (6)
- Hepatosplenic T-Cell Lymphoma (2)
- Hereditary Thyroid Gland Medullary Carcinoma (1)
- High Grade B-Cell Lymphoma (1)
- High Grade Ovarian Serous Adenocarcinoma (8)
- Hodgkin Lymphoma (9)
- Hypopharyngeal Squamous Cell Carcinoma (2)
- Infectious Mononucleosis (1)
- Intrahepatic Cholangiocarcinoma (6)
- Invasive Breast Carcinoma of No Special Type (12)
- Invasive Breast Lobular Carcinoma (1)
- Kidney Neoplasm (1)
- Kidney Rhabdoid Tumor (1)
- Krukenberg Tumor (1)
- Liposarcoma (1)
- Lung Adenocarcinoma (17)
- Lung Giant Cell Carcinoma (8)
- Lung Large Cell Carcinoma (9)
- Lung Mucoepidermoid Carcinoma (1)
- Lung Non-Small Cell Carcinoma (2)
- Lung Small Cell Carcinoma (25)
- Lung Squamous Cell Carcinoma (9)
- Lymphoblastic Lymphoma (1)
- Malignant Peripheral Nerve Sheath Tumor (1)
- Mantle Cell Lymphoma (5)
- Mature Gastric Teratoma (1)
- Maxillary Sinus Squamous Cell Carcinoma (1)
- Medaka Hepatoma (2)
- Medulloblastoma (3)
- Melanoma (24)
- Meningioma (2)
- Minimally Invasive Lung Adenocarcinoma (1)
- Monophasic Synovial Sarcoma (1)
- Mouse Bladder Transitional Cell Carcinoma (1)
- Mouse Chondrosarcoma (1)
- Mouse Colon Adenocarcinoma (3)
- Mouse Ependymoma (2)
- Mouse Erythroid Leukemia (13)
- Mouse Fibrosarcoma (5)
- Mouse Glioblastoma (1)
- Mouse Hemangioendothelioma (1)
- Mouse Hepatocellular Carcinoma (1)
- Mouse Insulinoma (3)
- Mouse Intestinal Tract Neuroendocrine Adenoma (1)
- Mouse Islet Cell Adenoma (1)
- Mouse Kidney Carcinoma (1)
- Mouse Leukemia (10)
- Mouse Leydig Cell Tumor (1)
- Mouse Lymphoma (8)
- Mouse Mammary Gland Malignant Neoplasm (23)
- Mouse Melanoma (9)
- Mouse Multiple Myeloma (5)
- Mouse Myeloid Leukemia (3)
- Mouse Neoplasm (1)
- Mouse Neuroblastoma (21)
- Mouse Oral Cavity Squamous Cell Carcinoma (1)
- Mouse Osteosarcoma (3)
- Mouse Pituitary Gland Neoplasm (1)
- Mouse Plasmacytoma (1)
- Mouse Precursor T Cell Lymphoblastic Lymphoma/Leukemia (2)
- Mouse Pulmonary Adenoma (1)
- Mouse Pulmonary Malignant Tumor (3)
- Mouse Pulmonary Squamous Cell Carcinoma (1)
- Mouse Rectum Carcinoma (2)
- Mouse Reticulum Cell Sarcoma (2)
- Mouse Sarcoma (1)
- Mouse Teratocarcinoma (8)
- Mouse Thymic Lymphoma (3)
- Mycosis Fungoides (1)
- Myelodysplastic Syndrome (1)
- Myxofibrosarcoma (1)
- Natural Killer Cell Lymphoblastic Leukemia/Lymphoma (2)
- Neuroblastoma (26)
- Oral Cavity Squamous Cell Carcinoma (15)
- Osteoid Osteoma (1)
- Osteosarcoma (15)
- Ovarian Carcinoma (1)
- Ovarian Clear Cell Adenocarcinoma (1)
- Ovarian Endometrioid Adenocarcinoma (4)
- Ovarian Granulosa Cell Tumor (1)
- Ovarian Mucinous Adenocarcinoma (2)
- Ovarian Serous Adenocarcinoma (2)
- Ovarian Serous Cystadenocarcinoma (2)
- Ovarian Small Cell Carcinoma (1)
- Pancreatic Adenocarcinoma (13)
- Pancreatic Carcinoma (5)
- Pancreatic Ductal Adenocarcinoma (12)
- Papillomavirus-Independent Cervical Squamous Cell Carcinoma (1)
- Papillomavirus-Related Cervical Adenocarcinoma (7)
- Papillomavirus-Related Cervical Squamous Cell Carcinoma (4)
- Papillomavirus-Related Endocervical Adenocarcinoma (16)
- Paroxysmal Nocturnal Hemoglobinuria (3)
- Pharyngeal Squamous Cell Carcinoma (1)
- Plasma Cell Myeloma (15)
- Pleural Epithelioid Mesothelioma (5)
- Pleural Sarcomatoid Mesothelioma (2)
- Poorly Differentiated Thyroid Gland Carcinoma (1)
- Primary Cutaneous T-Cell Non-Hodgkin Lymphoma (1)
- Primary Effusion Lymphoma (7)
- Primitive Neuroectodermal Tumor (1)
- Prostate carcinoma (1)
- Prostate Carcinoma (9)
- Rat C-Cell Carcinoma (1)
- Rat Cholangiocarcinoma (1)
- Rat Colon Adenocarcinoma (5)
- Rat Digestive System Neoplasm (1)
- Rat Fibrosarcoma (1)
- Rat Hepatocellular Carcinoma (20)
- Rat Histiocytic Sarcoma (1)
- Rat Insulinoma (2)
- Rat Leukemia (1)
- Rat Leydig Cell Adenoma (1)
- Rat Lung Carcinoma (1)
- Rat Malignant Glioma (4)
- Rat Malignant Meningioma (1)
- Rat Malignant Oligodendroglioma (2)
- Rat Malignant Thymoma (3)
- Rat Mammary Gland Adenocarcinoma (10)
- Rat Neuroblastoma (3)
- Rat Osteosarcoma (2)
- Rat Pituitary Gland Neoplasm (6)
- Rat Prostate Adenocarcinoma (3)
- Rat Rhabdomyosarcoma (1)
- Rat Sarcoma (2)
- Rat Squamous Cell Carcinoma (1)
- Rat Urinary Bladder Transitional Cell Carcinoma (2)
- Rat Urinary System Neoplasm (6)
- Rectal Adenocarcinoma (13)
- Rectosigmoid Adenocarcinoma (1)
- Recurrent Bladder Carcinoma (1)
- Renal Cell Carcinoma (7)
- Renal Pelvis Urothelial Carcinoma (1)
- Retinoblastoma (11)
- Sacral Chordoma (1)
- Sacrococcygeal Teratoma (1)
- Salivary Gland Squamous Cell Carcinoma (1)
- Sezary Syndrome (1)
- Shwachman-Diamond Syndrome (1)
- Skin Squamous Cell Carcinoma (2)
- Splenic Marginal Zone Lymphoma (1)
- Testicular Embryonal Carcinoma (8)
- Testicular Teratoma (2)
- Testicular Yolk Sac Tumor (1)
- Thyroid Gland Anaplastic Carcinoma (10)
- Thyroid Gland Follicular Carcinoma (4)
- Thyroid Gland Papillary Carcinoma (3)
- Thyroid Gland Sarcoma (1)
- Thyroid Gland Squamous Cell Carcinoma (2)
- Tongue Adenosquamous Carcinoma (1)
- Tongue Squamous Cell Carcinoma (6)
- Type I Endometrial Adenocarcinoma (1)
- Ureter Urothelial Carcinoma (1)
- Uterine Carcinosarcoma (2)
- Uterine Corpus Leiomyosarcoma (1)
- Uterine Corpus Sarcoma (2)
- Uveal Melanoma (2)
- Vaginal Melanoma (2)
- Vulvar Melanoma (1)
- Vulvar Squamous Cell Carcinoma (1)
Description: Established from the lymph node metastasis of a malignant melanoma from a 42-year-old Caucasian ...
Description: Established from the primary tumor of a 58-year-old woman with melanoma in 1977
Description: Established from the tumor biopsy of an intracranial secondary melanoma in a 28-year-old European ...
Description: Derived from tumor tissue (subcutis metastasis) of a 67-year-old Caucasian man with malignant ...
Description: Established from the subcutaneous nodule of a 14-year-old boy with melanoma in 1990
Description: Derived in 1992 from right axillary lymph nodes of a 43-year-old Caucasian man with metastatic ...
Description: Epstein-Barr virus-positive cell line established from peripheral blood in 1977 from male patient ...
Description: Established from the lymph node metastasis (groin) of a 26-year-old man with malignant melanoma ...
Description: Established from the primary (achromic) cutaneous tumor (left thigh) of a 26-year-old man with ...
Description: Established from the primary tumor (right cervical) of a 64-year-old woman with cutaneous melanoma
Description: Established in 1990 from the right axillary lymph node of a 48-year-old woman with metastatic ...
Description: Established in 1985 from the soft tissue metastasis of a malignant melanoma from a 47-year-old woman
Description: This lymphoblastoid cell line was established by EBV transformation of peripheral blood ...
Description: Human melanoma, spindle-shaped. Said DOPA (+). Cell growth is slow.
Description: Human malignant melanoma cell line with the production of 5-S-cysteinyldopa.
Description: malignant melanoma, metastasis to lymph node