H9C2(2-1)
Cat.No.: CSC-C9387L
Species: Rattus norvegicus (Rat)
Source: Embryo; Heart
Morphology: Myoblast
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The H9C2(2-1) cell line is an immortalized rat cardiomyoblast line that has been extensively used as a cardiomyocyte model. They were first isolated in 1976 from embryonic BDIX rat ventricular tissue by Kimes and Brandt. The cells exhibit fibroblast-like morphology when they remain undifferentiated. H9C2(2-1) cells can be partially differentiated into cardiac-like myotubes under low-serum culture conditions or with treatment with retinoic acid and can express markers of mature myocardial cells including troponin T, α-actinin, and myosin heavy chain. Although the cells do not completely recapitulate the mature electrophysiological phenotype of cardiomyocytes, H9C2(2-1) cells still express functional ion channels such as L-type calcium and sodium channels and exhibit responses to β-adrenergic stimulation.
H9C2(2-1) cells are frequently used in the study of myocardial ischemia-reperfusion injury, hypertrophy, and drug-induced cardiotoxicity. Additionally, they are a more cost-effective and easily cultivable alternative to primary cardiomyocytes and they are easily transfected, allowing for genetic manipulations to study mechanisms.
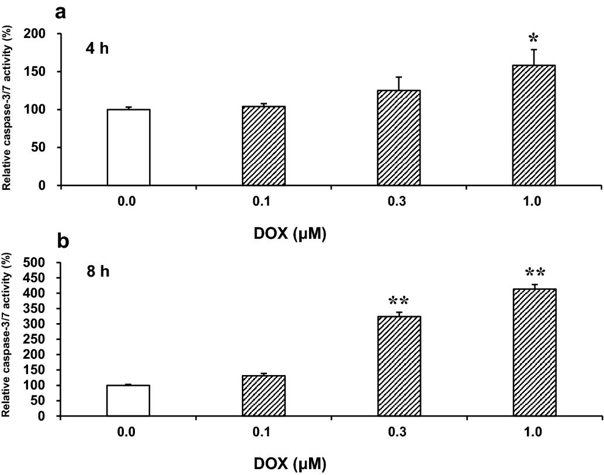
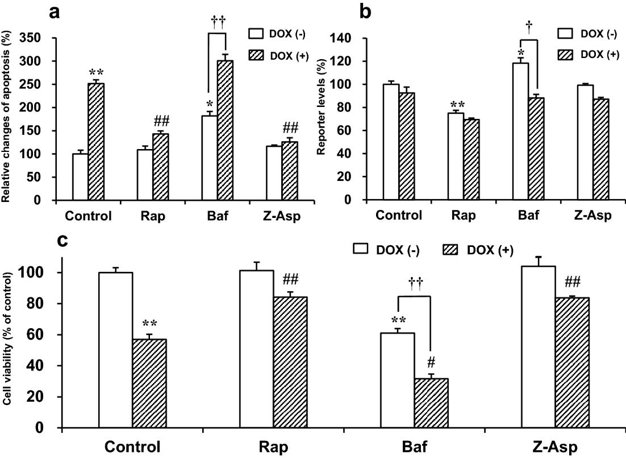
Effects of Rap, Baf, or Z-Asp on Apoptosis, Autophagy, and Cell Viability in DOX-Treated H9c2(2-1) Cells
Doxorubicin (DOX) is a potent chemotherapeutic agent; however, it causes severe heart injury via apoptosis induction in many patients. DOX-induced cardiotoxicity is attenuated by activated autophagy in the heart. Kanno et al. previously found that programmed cell death 1 (Pdcd1), an immune checkpoint receptor, inhibits DOX-induced cardiomyocyte apoptosis. In this study, they examined whether autophagy is involved in the anti-apoptotic effect of Pdcd1 against DOX.
DOX activates the executioner caspases-3/7, the key mediators of apoptosis. First, in a preliminary experiment, they determined the time-course of caspase-3/7 activation in H9c2(2-1) cells treated with 0.1-1 μM DOX (Fig. 1). These DOX concentrations were in the range of physiologically relevant cardiotoxic concentrations. At 4 h, significant caspase activation was detected with 1 μM, and at 8 h with ≥0.3 μM DOX. Therefore, they chose 1 μM DOX for further experiments to ensure early caspase activation. In Fig. 2, they showed the effects of Rap (autophagy inducer), Baf (autophagy inhibitor), and Z-Asp (pan-caspase inhibitor) on apoptosis, autophagy, and cell viability in DOX-treated H9c2(2-1) cells. Without any of these modulators, DOX (1 μM) robustly induced apoptosis after 8 h and decreased cell viability after 18 h. Interestingly, viability only decreased after the onset of apoptosis. The rate of apoptosis and cell viability in DOX-treated cells was 251.4% and 57.0% of that of untreated cells, respectively (Fig. 2a, c), suggesting that DOX initiates caspase-dependent apoptosis and subsequently induces cell death.
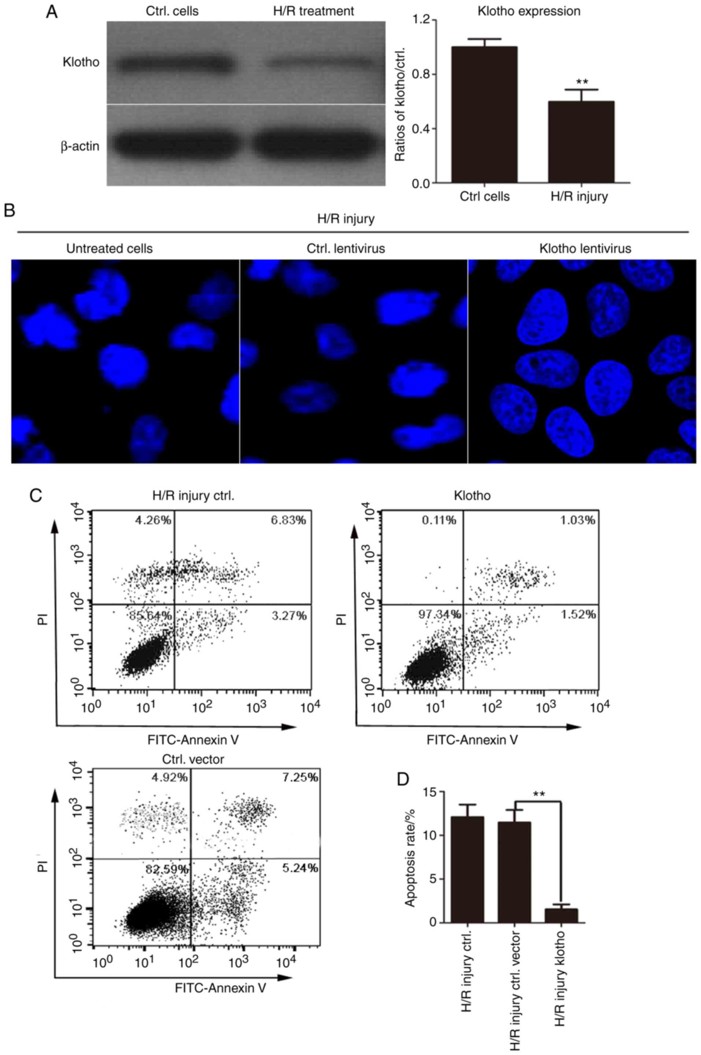
Overexpression Of Klotho Markedly Inhibits the H/R-Induced Apoptosis of H9C2(2-1) Cells
Early reperfusion is the most effective and important treatment for acute myocardial infarction. However, reperfusion therapy is often associated with a certain degree of myocardial damage. In the present study, Hu's team aims to identify the role of klotho, and the molecular mechanism underlying its effects, in myocardial damage in a model of myocardial hypoxia injury.
They first detected klotho protein levels by western blotting in H9c2(2-1) cells after H/R. As shown in Fig. 3A, H/R injury significantly reduced klotho expression. To further explore the role of klotho in apoptosis, they performed Hoechst 33258 nuclear staining. H/R-treated cells showed nuclear shrinkage, condensed chromatin with bright fluorescence, nuclear fragmentation, and apoptotic bodies. However, klotho-overexpressing cells had smooth, intact, and uniformly stained nuclei, which suggested that klotho overexpression alleviated H/R-induced apoptosis (Fig. 3B). To quantitatively detect the anti-apoptotic effect of klotho, H9c2(2-1) cells were infected with klotho lentivirus or control vector before H/R exposure, and apoptosis was measured by Annexin V-FITC/PI staining. As shown in Fig. 3C, H/R significantly increased apoptosis in control cells, but klotho overexpression markedly reduced the apoptotic rate. The results demonstrate that klotho inhibited H/R-induced apoptosis in H9c2(2-1) cells.
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells