How to Maximize Efficiency in Cell-Based High-Throughput Screening?
Cell-based High-Throughput Screening (HTS) is a crucial technique for drug discovery and cell biology research, allowing for high-throughput analysis and screening of cell responses to various compounds and experimental conditions. With the use of cell-based assays, it is possible to rapidly screen large compound libraries, providing valuable information on cell viability, gene expression, and protein activity. This enables high-speed, cost-effective initial toxicity screening, target validation, and compound optimization.
The Common Workflow of Cell-Based HTS Experiments
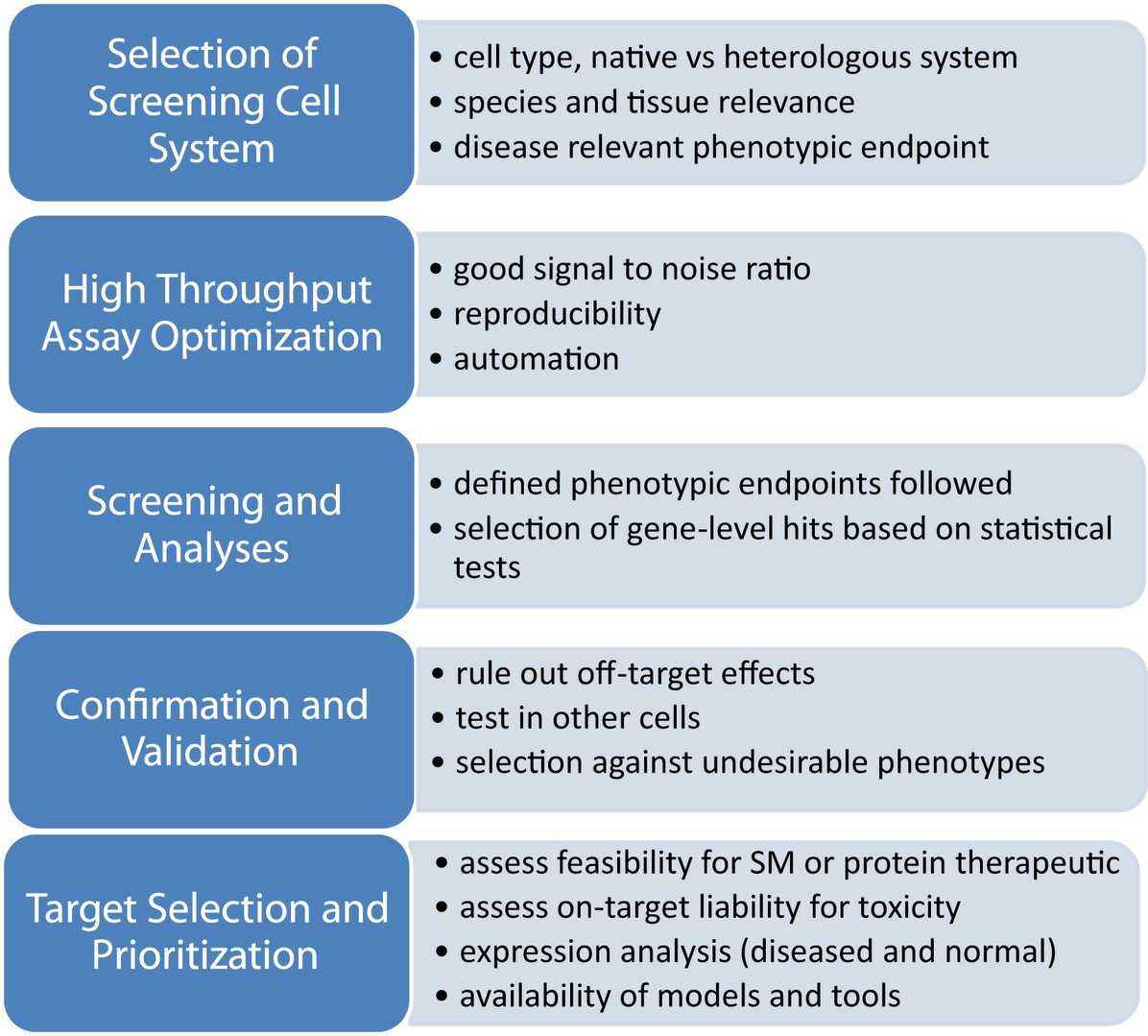 Fig. 1. Generic Flow Scheme for Cell-Based High-Throughput Screening (Finkbeiner S, Frumkin M, et al., 2015).
Fig. 1. Generic Flow Scheme for Cell-Based High-Throughput Screening (Finkbeiner S, Frumkin M, et al., 2015).
1. Experimental Design and Preparation
Prior to the experiment, screening objectives and experimental design must be defined. This includes selecting appropriate cell lines, determining assay endpoints (e.g., cell viability, gene expression, protein activity), choosing compound libraries, and establishing experimental conditions (e.g., concentration, incubation time).
2. Cell Seeding and Culture
Cells are cultured in appropriate 96-, 384- or 1536-well plates to facilitate high-throughput processing. The seeding density, culture duration, and any other pre-treatment requirements (e.g. pre-treatment with IFN-γ) should be strictly controlled.
3. Compound Library Addition and Incubation
Compounds are added to the assay plates from stock solutions, which are typically handled with automated liquid handling systems. The incubation time is optimized for the specific target (usually 24-72 hours), and the plates should be kept still for as long as possible during this period to avoid edge effects.
4. Detection and Signal Measurement
Detection reagents specific for the screening purpose are added to the assay plates, such as fluorescent, luminescent or colorimetric substrates. Detection instruments for high-throughput screening (e.g. multi-mode plate readers, plate-based imaging systems, high-content analyzers) are then used to measure signals.
5. Data Analysis
Raw data should be normalized, and the Z'-factor calculated for quality control of the assay. Preliminary active compounds ("hits") can be identified and statistical analysis and data visualization performed.
6. Hit Confirmation and Re-Screening
Replicate experiments or secondary screening can be performed for the primary hit compounds to confirm activity and specificity, and eliminate false positives.
Best Practices in Assay Development
Cell model selection:
Choosing the right cell model is crucial for assay success:
- Primary Cells: Best for reflecting physiological states but have limited availability and significant batch variability.
- Cell Lines: Offer stability, but may undergo genotypic/phenotypic drift over time.
- Engineered Cell Lines: Use reporter systems for real-time monitoring but require verification to ensure no mutations or off-target effects.
Optimization of experimental conditions:
Optimization includes media and reagents, incubation time and temperature, and detection parameters.
- Media and Reagents: Select growth-appropriate media and ensure reagent stability. For example, DMSO (Dimethyl Sulfoxide) is commonly used to enhance compound solubility, but its cytotoxicity needs to be controlled.
- Incubation Time and Temperature: Different assays may require different incubation times (e.g., 48h or 72h) to observe cellular responses. Temperature is typically maintained at 37°C to mimic the in vivo environment.
- Detection Parameters: Choose suitable detection parameters based on the research objective, such as cell viability, fluorescence signal, enzyme activity, or apoptosis rate. For instance, using fluorescent reporter genes (e.g., SEAP, GFP) enables real-time monitoring of cellular states.
Microplate format optimization:
Selecting an optimal plate format improves both efficiency and data quality:
- Plate Type: Choose between 96-well, 384-well, or 1536-well plates based on throughput requirements. For high-content screening (HCS), clear-bottom plates are recommended for imaging purposes.
- Layout Optimization: To avoid edge effects, the wells along the border of the plate could be filled with buffer or assigned as controls; ensure positive, negative and blank controls are evenly distributed across the plate to reduce local variation and ensure data accuracy.
- Cell Seeding Density: The seeding density of cells could be optimized to ensure consistent and uniform number of cells across wells, thereby reducing data variability.
- Detection Method Compatibility: Choose black or white plates (black reduces light scattering, white enhances signal reflection) and ensure plate material is compatible with the detection wavelengths to avoid issues such as autofluorescence. If the detection method involves multiplexed detection (e.g., viability, calcium flux, membrane potential), then choose a plate format that allows multi-wavelength detection.
Data processing and analysis:
- Data Processing and Normalization: Data quality control is critical in HTS, to assess the assay reliability. Assay replicates and the inclusion of negative and positive controls allow to assess the robustness of the data. In addition, the statistical parameter Z-factor, defined as, can be used to determine the signal-to-noise ratio and the suitability of the assay for HTS. Z-factors close to 1 indicate a high signal-to-noise ratio and a more suitable assay for HTS screening.
- Minimizing False Positives and False Negatives: False positives and false negatives are important issues in HTS and can be addressed with appropriate controls. To reduce false positives, several rounds of validation are recommended, including independent replicates, validation across multiple concentration gradients, and orthogonal assay confirmation. Statistical analysis (e.g ., ANOVA) can also be used to identify systematic errors in the data, improving the data reliability.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
| Cell-based Screening and Profiling Services | Speed up your drug discovery with Creative Bioarray's cell-based screening and profiling services. |
Reference
- Finkbeiner S, Frumkin M, et al. Cell-based screening: extracting meaning from complex data. Neuron. 2015. 86(1):160-74.