Summary of Approaches for Loading Cargo into Exosomes
Exosomes are small extracellular vesicles released by cells into the extracellular space. They are enriched in various biomolecules, including proteins, lipids, and nucleic acids, which can be harnessed for therapeutic purposes. To fully exploit the potential of exosomes as therapeutic vehicles, it is crucial to efficiently load them with specific cargo molecules. This enables targeted delivery and controlled release of therapeutic agents to desired cell types or tissues.
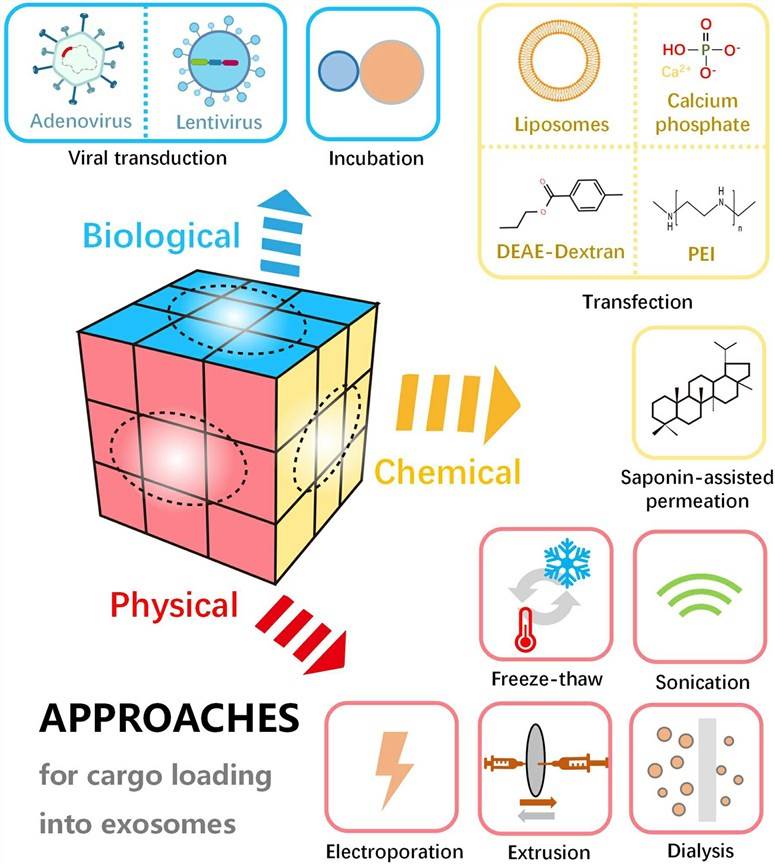 Fig. 1 Schematic diagram of various approaches for cargo loading into or onto exosomes. (Chen H, et al., 2021)
Fig. 1 Schematic diagram of various approaches for cargo loading into or onto exosomes. (Chen H, et al., 2021)
Physical Methods
- Sonication
Sonication is a physical method commonly used to load cargo into exosomes. In this approach, exosomes are subjected to high-frequency sound waves, which generate mechanical forces that disrupt the exosome membrane and create transient pores. Cargo molecules present in the surrounding solution can then diffuse into the exosome interior through these pores. Sonication can efficiently load various cargos, including small molecules, proteins, and nucleic acids, into exosomes. - Electroporation
Electroporation involves the application of short electrical pulses to create temporary pores in the exosome membrane, allowing cargo molecules to enter the exosome. By adjusting the pulse parameters, the size and number of pores can be controlled to optimize cargo loading efficiency. Electroporation is particularly effective for loading large molecules, such as plasmid DNA or siRNA, into exosomes. - Freeze-thaw
Freeze-thaw cycles can be employed to load cargo into exosomes. In this method, exosomes are subjected to repeated cycles of freezing and thawing, which disrupts the exosome membrane and promotes cargo incorporation. The freeze-thaw method is simple, cost-effective, and compatible with various cargo types. However, it may affect the stability and integrity of exosomes to some extent. - Extrusion
Extrusion involves the passage of exosome suspension through a porous membrane under controlled pressure. This process leads to the formation of smaller-sized exosomes and facilitates the incorporation of cargo molecules. Extrusion can be performed using specialized extrusion devices or syringe filters with defined pore sizes. This method allows for efficient loading of cargo while preserving the structural integrity of exosomes.
Chemical Methods
- Saponin-assisted permeation
Saponins are natural surfactant compounds that can transiently disrupt the exosome membrane, facilitating the entry of cargo molecules. Saponin-assisted permeation involves the incubation of exosomes with saponins, followed by the addition of cargo molecules. The exosomes are then subjected to a subsequent purification step to remove excess saponins and unbound cargo. This method is particularly useful for loading hydrophobic molecules into exosomes. - Transfection reagent-based encapsulation
Transfection reagents, commonly used for intracellular nucleic acid delivery, can also be employed for exosome cargo loading. In this approach, cargo molecules are complexed with transfection reagents, forming nanoparticles that can be incorporated into exosomes. The transfection reagent-based encapsulation method offers versatility in cargo types and efficient loading of both hydrophilic and hydrophobic molecules into exosomes.
Biological Methods
- Incubation
Incubation involves co-culturing exosomes with cells or tissues in the presence of cargo molecules. During the incubation period, cargo molecules can naturally enter the exosome via passive diffusion, endocytosis, or other cellular uptake mechanisms. This method allows for the loading of various cargo types without the need for additional manipulation steps. However, the efficiency of cargo loading through incubation may vary depending on the cargo properties and the affinity of exosomes for the cargo molecules. - Viral transduction-based strategies
Viral transduction-based techniques employ viral vectors to genetically engineer donor cells for generating exosomes that contain particular cargo molecules. The donor cells express the cargo molecules that are thereafter packaged into exosomes during their biogenesis. This methodology enables the production of exosomes having high efficacy and specificity concerning cargo loading. Nevertheless, it necessitates the use of viral vectors and genetic manipulation of the cells.
Creative Bioarray Relevant Recommendations
Creative Bioarray provides the best quality lyophilized exosome standards and fluorescent exosome standards obtained from several biological samples, including hundreds of different cell lines, plasma, serum, saliva, and urine as well as other bio-fluids.
Reference
- Chen H, et al. (2021). "Exosomes, a New Star for Targeted Delivery." Front Cell Dev Biol. 9, 751079.