Plasma Protein Binding Assay
- Service Details
- Features
- FAQ
- Explore Other Options
Plasma protein binding (PPB) is a critical determinant of drug disposition, efficacy, and safety. Only the unbound fraction of a drug is pharmacologically active and able to reach its target site, making accurate assessment of PPB essential throughout discovery and development. Based on the free drug theory, the concentration of unbound drug in plasma reflects its availability in tissues, and thus directly influences pharmacokinetic/pharmacodynamic (PK/PD) relationships.
Even small variations in binding can alter systemic exposure, precipitate drug–drug interactions, or increase the risk of toxicity, especially when displacement occurs or protein levels change in disease states. Cross-species PPB data also play a vital role in predicting human responses, defining safety margins, and informing first-in-human (FIH) dose selection. Accurate PPB analysis is therefore not only a regulatory requirement but also a strategic decision-making tool that reduces development risk and accelerates the path from discovery to clinic.
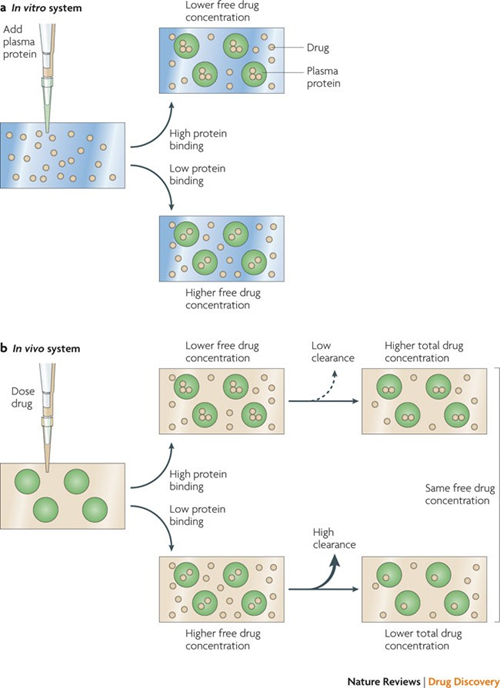 Fig. 1. Effects of plasma protein binding on in vitro and in vivo systems (Smith D A, Di L, et al., 2010).
Fig. 1. Effects of plasma protein binding on in vitro and in vivo systems (Smith D A, Di L, et al., 2010).
Our Methods for Plasma Protein Binding
Equilibrium dialysis (the gold standard)
The RED Device is comprised of two chambers separated by a semi permeable membrane. The drug-spiked plasma sample is placed in one chamber, with a buffer solution in the other. Free, unbound drug (with a low molecular weight) is able to diffuse through the membrane to the other side. Once equilibrium has been reached, typically after 4-6 hours, the concentrations in each chamber are measured, providing a quantitative measurement of the binding percentage. Large molecules such as plasma proteins and bound drug molecules are unable to pass through the membrane, and remain in the plasma chamber.
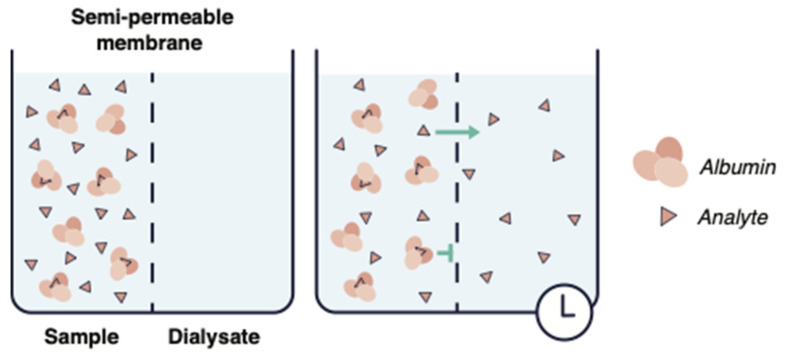 Fig. 2. Illustration of Rapid Equilibration Dialysis (RED) (Ahmed H, Bergmann F, et al., 2022).
Fig. 2. Illustration of Rapid Equilibration Dialysis (RED) (Ahmed H, Bergmann F, et al., 2022).
Advantages of the RED Technology:
- High-Throughput: 96 well plate format accommodates large numbers of samples in parallel, significantly reducing project time.
- High Efficiency: It achieves equilibrium rapidly, reducing the overall assay time.
- High Accuracy: Non-specific binding and sample dilution concerns associated with alternative methods are minimized, providing results that are more representative of in vivo performance.
Ultrafiltration
Ultrafiltration (UF) separates free drug from bound by centrifugal filtration through a membrane. UF is fast and amenable to high-throughput formats, making it a useful tool for screening applications. Artifacts from nonspecific binding to the filter membranes, centrifugation speed, sample temperature, and concentration polarization can all influence results. We apply UF for high-volume screening and when sample throughput constraints require it.
Ultracentrifugation
Ultracentrifugation separates free drug from protein-bound species by density and sedimentation; it avoids membrane contact and is less prone to filter adsorption artifacts. UC is used when high accuracy binding constants are needed, or with problematic compounds.
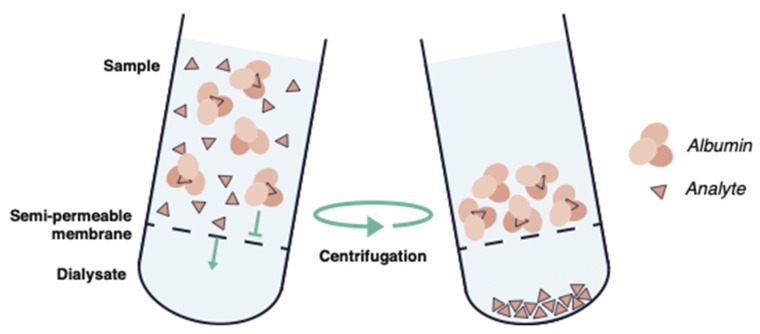 Fig. 3. Schematic illustration of ultracentrifugation (Ahmed H, Bergmann F, et al., 2022).
Fig. 3. Schematic illustration of ultracentrifugation (Ahmed H, Bergmann F, et al., 2022).
| Species | Human (pooled or individual plasma), rat, mouse, dog, monkey, minipig; others upon request |
| Analytical Method | Quantification by LC-MS/MS |
| Replicates | Typically triplicate per test condition to ensure accuracy and reproducibility |
| Deliverables | % Plasma Protein Binding, unbound fraction (fu,p), raw LC-MS/MS data, full study report, and data tables |
Why Choose Our PPB Services?

Robust Quality Control
We perform thorough method validation for each project, including assessments of equilibrium time, sample stability, and recovery, ensuring the reliability and reproducibility of our data.
Comprehensive Species Options
We offer PPB determination in a wide range of species, including mouse, rat, dog, non-human primate, and human plasma, supporting your research from preclinical to clinical stages.
Efficient Project Delivery
Our optimized RED platform enables us to deliver high-quality reports in a timely manner.

Expert Scientific Support
Our team of experienced DMPK scientists provides professional guidance on experimental design and data interpretation, helping you make informed decisions throughout your drug development journey.
FAQ
Why is it so important to measure plasma protein binding?
PPB is a key factor in determining a drug's potency, toxicity and half-life, and ultimately its suitability as a drug candidate. Only the unbound fraction of a drug is active, and so knowing and being able to accurately measure this important parameter will help determine what a drug's true behavior in vivo will be. For that reason, it is a very important step in the preclinical research process.
Which method should I choose for my program — equilibrium dialysis or ultrafiltration?
For most discovery and regulatory needs, we recommend equilibrium dialysis (RED) as the primary method because it provides the most robust estimate of thermodynamic free fraction across diverse chemistries. Ultrafiltration is a good method for high-throughput screening purposes; however, filter binding and centrifugation conditions need to be carefully controlled.
Can you determine if there is concentration-dependent binding?
Yes. By testing at multiple concentrations, we can evaluate non-linear binding and identify potential saturation effects.
Do you report fu values in matrices other than plasma (e.g. microsomes, brain homogenates)?
Yes. We have methods available for unbound fraction determination in microsomes, S9 fractions and tissue homogenates, which can be used to support IVIVC and PBPK modeling.
What factors can affect plasma protein binding?
Binding can be affected by a number of factors, including drug concentration (particularly if you have saturable binding sites), plasma protein concentration, disease states (such as liver or kidney disease which may impact the levels of binding proteins), drug-drug interactions (competitive binding), and environmental factors such as pH and temperature. The conditions we run our assays under are physiologic to most closely mimic the in vivo conditions.
References
- Ahmed H, Bergmann F, et al. Protein Binding in Translational Antimicrobial Development-Focus on Interspecies Differences. Antibiotics. 2022; 11(7):923.
- Smith D A, Di L, et al. The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat Rev Drug Discov. 2010. 9, 929–939
Explore Other Options