From Primary to Immortalized: Navigating Key Cell Lines in Biomedical Research
Primary Cell Lines
Primary cell lines represent cells which researchers obtain directly from animal or human tissues. These cells maintain their original tissue characteristics while having restricted cell division abilities. Serial passaging leads to primary cells aging until they reach senescence or die.
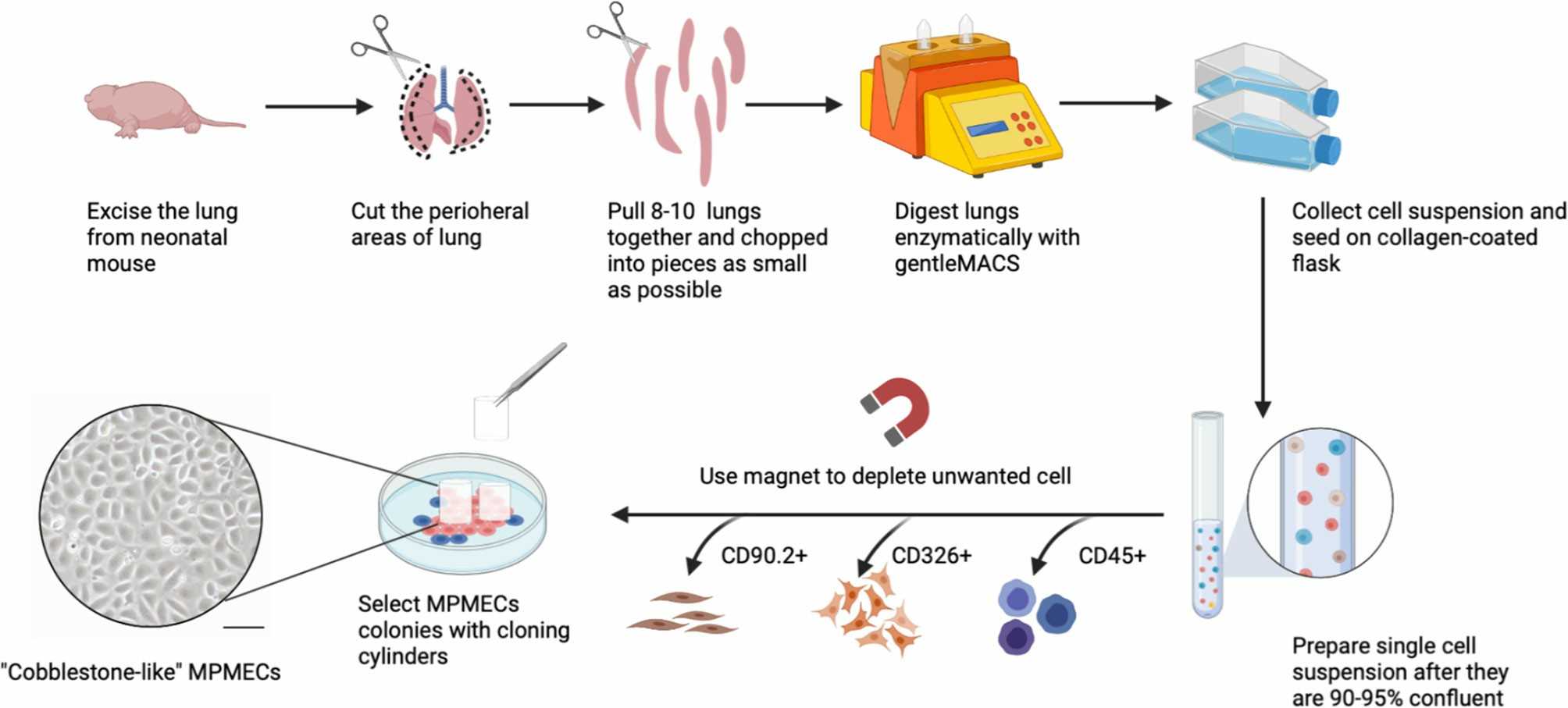 Fig. 1. Schematic representation of primary cell isolation (mouse pulmonary microvascular endothelial cell as example) (Liu X, Xia F, et al., 2021).
Fig. 1. Schematic representation of primary cell isolation (mouse pulmonary microvascular endothelial cell as example) (Liu X, Xia F, et al., 2021).
Stem Cell Lines
Stem cells are self-regenerating, diversely differentiated cells that originate most commonly from umbilical cord blood, embryos, bone marrow, fat tissue and peripheral blood. They can be cultivated and differentiated into human tissues, including muscle, nerve and bone. Among them, embryonic stem cells, mesenchymal stem cells and induced pluripotent stem cells are the best-studied and widely used types, possessing immense utility and usefulness.
Embryonic stem cells (ESCs)
Embryonic Stem Cells (ESCs): ESCs are derived from an inner mass of a blastocyst or early embryo and have the capacity to differentiate into nearly any type of human cell. Additionally, they can easily proliferate in vitro indefinitely.
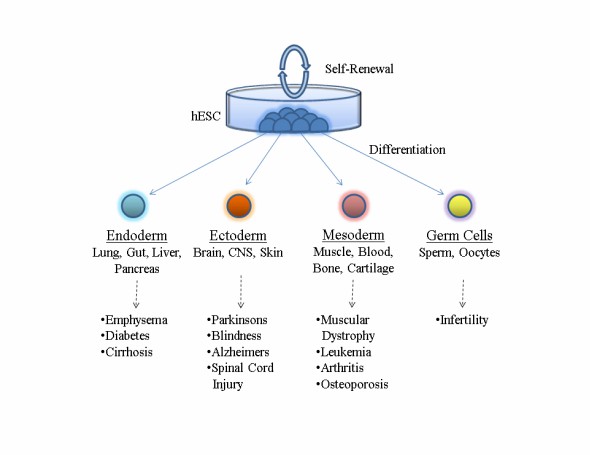 Fig. 2. The differentiation potential of human embryonic stem cells and examples of tissue specific diseases that are being investigated (Heindryckx B, Leary TO, et al., 2012).
Fig. 2. The differentiation potential of human embryonic stem cells and examples of tissue specific diseases that are being investigated (Heindryckx B, Leary TO, et al., 2012).
Generation of Human ES Cells
Table 1. Advantages and disadvantages of human ES cell derivation methods (Moon SY, Park YB, et al., 2006)
| Derivation method | Advantages | Disadvantages |
| Partial embryo culture method | Efficient for the derivation of human | Difficult to isolate the portion of the blastocyst containing the ICM |
| ES cells from blastocysts possessing | ||
| large or small ICMs | ||
| Immunosurgical method | Easy isolation of the ICM from blastocysts possessing large and distinct ICMs | Possibility of contamination with animal pathogens |
| Selective removal of the trophectoderm from expanded blastocysts | Difficult to isolate ICM from blastocysts having small or indistinct ICMs | |
| Whole embryo culture method | Can be used regardless of blastocyst quality | Risk of trophectodermal overgrowth, which impedes the growth of the ICM |
| No loss of ICMs during the process | ||
| Alleviates contamination due to animal pathogens |
Adult stem cells
Adult stem cells are undifferentiated cells that exist in tissues and organs including skin, fat tissue, muscle and bone marrow.
Hematopoietic Stem Cells (HSCs)
HSCs is a type of adult stem cell with high self-renewal and multipotent differentiation capabilities. These cells primarily originate from bone marrow, peripheral blood, and umbilical cord blood, and are the progenitor cells for all blood cells and immune cells within the hematopoietic system. They play a crucial role in maintaining blood homeostasis and repairing damaged tissues.
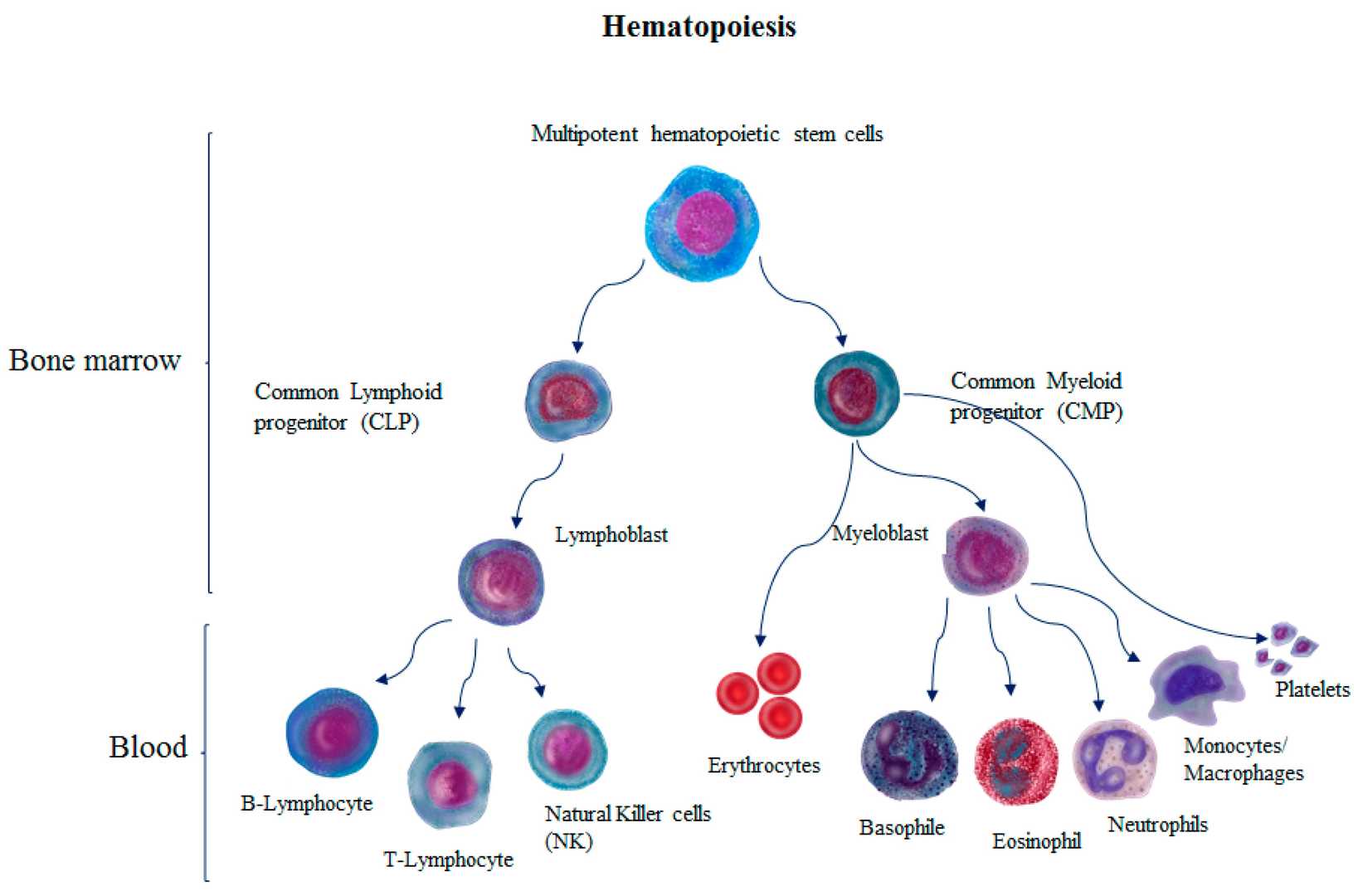 Fig. 3. Hematopoiesis process (Alomari M, Almohazey D, et al., 2019).
Fig. 3. Hematopoiesis process (Alomari M, Almohazey D, et al., 2019).
MSCs are adult multipotent stem cells with self-renewal and multi-lineage differentiation potential, that primarily originate from bone marrow, fat tissue and umbilical cord blood. They are the most broadly used adult stem cells at present.
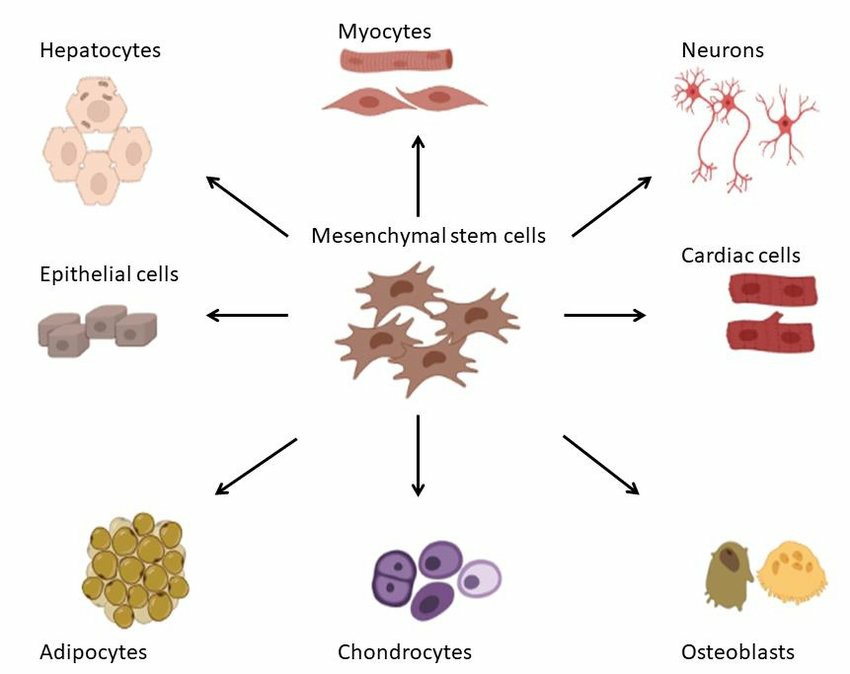 Fig. 4. The differentiation potential of mesenchymal stem cells (Dzobo K, 2021).
Fig. 4. The differentiation potential of mesenchymal stem cells (Dzobo K, 2021).
Neural Stem Cells (NSCs):
NSCs are unipotent adult stem cells that can convert into basic nervous system cell types such as neurons, astrocytes and oligodendrocytes. In early embryos, they arise predominantly from the neural plate and neural tube; in adults, they occur mostly in regions of the brain called the subventricular zone and the subgranular zone of the hippocampal dentate gyrus.
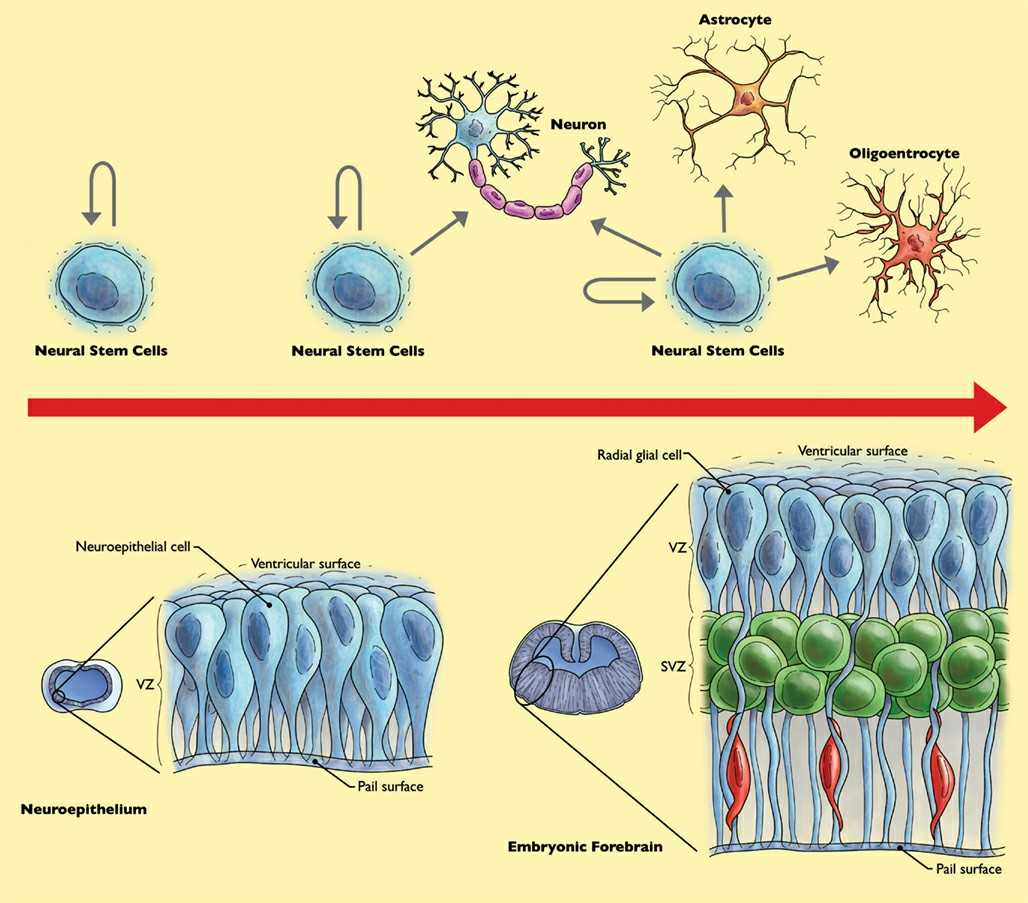 Fig. 5. Behavior of neural stem cells (NSC) throughout forebrain development (Olynik BM and Rastegar M, 2012).
Fig. 5. Behavior of neural stem cells (NSC) throughout forebrain development (Olynik BM and Rastegar M, 2012).
Induced pluripotent stem cells (iPSCs)
iPSCs are pluripotent stem cells reprogrammed from adult cells through the use of genetic engineering protocols to possess embryonic stem cell properties. iPSCs have many advantages over embryonic stem cells, including no ethical controversies, mass procurement and personalized therapy. But low reprogramming performance, safety and differentiation control concerns all need to be addressed.
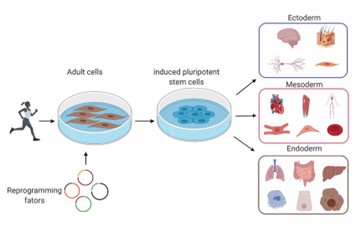 Fig. 6. Induced pluripotent stem cells are reprogrammed adult human cells from patients or healthy individuals (Delsing L, Herland A, et al., 2020).
Fig. 6. Induced pluripotent stem cells are reprogrammed adult human cells from patients or healthy individuals (Delsing L, Herland A, et al., 2020).
Generation of iPSCs:
- Cell Source: iPSCs can be derived from skin fibroblasts, hepatocytes, gastric cells, bone marrow cells, etc.
- Transfection of Reprogramming Factors: Key transcription factors (Oct4, Sox2, Klf4, and c-Myc) are introduced into target cells via viral transfection, plasmid electroporation, or small molecule induction to reacquire pluripotency.
- Cultivation and Characterization: Transfected cells are cultured in an appropriate medium and their pluripotency is confirmed by detecting cell surface markers and gene expression profiles.
Differentiation of iPSCs:
- Formation of Embryoid Bodies (EBs): EBs are 3D structures analogous to early embryos, within which iPSCs self-organize and differentiate into various cell types of embryonic development.
- Differentiation into Specific Lineages: iPSCs can be differentiated into specific lineages, such as neural cells, cardiomyocytes, and hepatocytes, by controlling various growth factors and small molecules.
- Maturation and Functional Validation: The differentiation process is tested based on the differentiated cell type to ensure they have normal physiological properties. For instance, the maturation of neurons could be monitored through electrophysiological activity and gene expression; the maturation of cardiomyocytes could be tracked via contractility and expression of specific biomarkers.
Tumor Cell Lines
The cells that form the bulk of tumors can be classified into both benign and malignant types. Benign tumor cells are usually not pathogenic, but malignant tumor cells can be invading and metastatic, able to penetrate the basement membrane and migrate to distant organs.
Primary tumor cells
Specifically, these are purified from tumor tissues (human, mouse, rat, rabbit) and isolated as single cells using protease or other techniques, then cultured in vitro to mimic in vivo conditions. They retain certain original characteristics of the tumor tissues.
Advantages: Close to patient tissue characteristics. Disadvantages: Difficult to isolate.
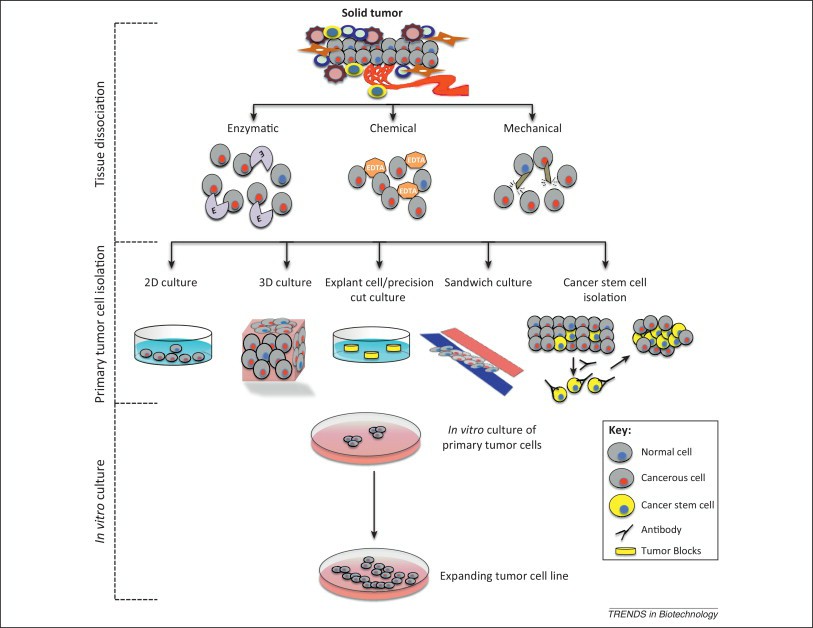 Fig. 7. A schematic representation of the process of primary tumor cell line establishment (Mitra A, Mishra L, et al., 2013).
Fig. 7. A schematic representation of the process of primary tumor cell line establishment (Mitra A, Mishra L, et al., 2013).
When primary cultured tumor cells can be continuously passaged for more than six months, grow stably, and retain the characteristics of tumor cells, they are termed tumor cell lines. As primary cells, they may develop genetic alterations or phenotype shifts to fit in vitro environments.
Advantages: Easy to handle, unlimited self-replicative potential, high homogeneity. Disadvantages: Genetic mutations and phenotypic drift.
CSCs are a small subpopulation of undifferentiated cell populations found in tumor tissues that are highly self-renewing and tumorigenic. They are responsible for the development, progression and recurrence of tumors and often demonstrate resistance to standard therapies. Other therapies that target CSCs include immunotherapy, small interfering RNA delivery, and gene knockout.
Circulating tumor cells (CTCs)
CTCs are cells excreted from primary or metastatic tumors, and migrate into the peripheral bloodstream. They can float in the blood and, eventually, invade far-off tissue, resulting in new metastatic lesions and cancer metastases.
Immune Cell Lines
Immune cells are a vital component of the human immune system, responsible for identifying and eliminating pathogens and abnormal cells, thereby maintaining the body's homeostasis.
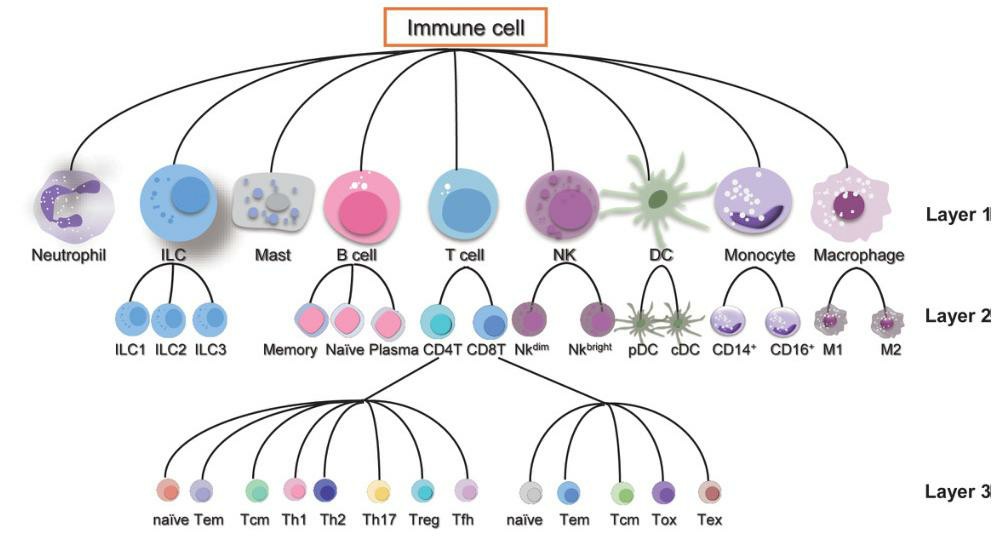 Fig. 8. Immune cell types and layer (Jiang Y, Chen Z, et al., 2023).
Fig. 8. Immune cell types and layer (Jiang Y, Chen Z, et al., 2023).
Classification of immune cells:
Innate immune cells:
- Monocyte-Macrophages: Monocytes in the blood migrate into tissues and differentiate into macrophages. They have strong phagocytic properties, feeding on and eliminating bacteria, old cells and cell debris. Moreover, macrophages process antigens and introduce antigen information to T cells that trigger particular immune reactions.
- Dendritic Cells (DCs): Proximally distributed, able to recognize, degrade and deliver antigens, DCs are critical cells in the development of adaptive immune responses.
- Natural Killer (NK) Cells: Directly recognize and kill infected or tumor cells, rapidly respond, and eliminate abnormal cells.
- Granulocytes: Including neutrophils, eosinophils, and basophils, these cells mainly participate in acute inflammatory responses and combat parasitic infections.
Adaptive immune cells:
- T Lymphocytes: Organized into helper T cells (Th), cytotoxic T cells (Tc) and regulatory T cells (Treg), these cells function as immune regulators, killing target cells directly and preserving immune tolerance.
- B Lymphocytes: Participate in humoral immune responses by secreting antibodies to neutralize pathogens.
- Memory Cells: These include memory T cells and memory B cells, which respond rapidly and efficiently to a new antigen exposure.
Immortalized Cell Lines
Immortalized cell lines are cell populations that have acquired the ability to continuously proliferate through certain means, allowing them to be passaged indefinitely without entering senescence. The high homogeneity and stability of these cell lines make them essential for research on cell growth, metabolic processes, and cancer formation.
Source
- Spontaneous Immortalization: Certain cancer cells possess the natural ability to achieve cellular immortality.
- Artificial Immortalization: Genetic engineering enables cells to gain unlimited proliferative potential through modifications such as telomerase reverse transcriptase addition or SV40 large T antigen expression.
- Virus-Induced: Polycyclic aromatic hydrocarbons induced hamster fibroblasts represent viral vectors that scientists can use for cell immortalization.
Characteristics
- Unlimited Proliferative Ability: Their ability to proliferate indefinitely allows these cells to survive and remain viable for long durations in laboratory cultures.
- Genetic Stability: Immortalized cell lines maintain stable phenotypes and gene expression patterns but might experience functional losses from genetic mutations under certain conditions.
- Altered Biological Characteristics: Immortalized cells often show biological properties that differ from in vivo cells which include variations in their differentiation levels and gene expression profiles.
- Good Experimental Reproducibility: The cells provide reliable experimental results because they can be cultured continuously without limitation.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
| Primary Cells | Creative Bioarray is your partner for Primary Cell Research. |
| Tumor Cells | Creative Bioarray offers 2,000+ tumor cell lines from human and animals in stock. All the tumor cell lines are comprehensively qualified by testing microbial contamination, virus contamination, and cross-culture contamination. |
| Immortalized Cells | Creative Bioarray offers a wide range of immortalized cell products, including human, murine, and other species-specific cell lines, as well as derivatives, immortalization kits, and extracellular vesicles. Designed for drug discovery, cancer research, regenerative medicine research, and more, our products provide consistent, scalable, and reproducible results with significant advantages over primary and tumor cells. |
| Stem Cell | Our tools for stem cell culture, differentiation, reprogramming, and analysis will remove technical barriers to move your experiments forward. |
| Stem Cell Research | Creative Bioarray offers a set of intelligent stem cell research to our global clients. |
| Cell Immortalization Service | Reliable and risk-free cell immortalization from fibroblasts, epithelial cells, muscle cells from human, rat, mouse, porcine and bovine. |
References
- Liu X, Xia F, et al. Isolation of Primary Mouse Pulmonary Microvascular Endothelial Cells and Generation of an Immortalized Cell Line to Obtain Sufficient Extracellular Vesicles. Front Immunol. 2021. 12:759176.
- Olynik BM, Rastegar M. The genetic and epigenetic journey of embryonic stem cells into mature neural cells. Front Genet. 2012. 3:81.
- Moon SY, Park YB, et al. Generation, culture, and differentiation of human embryonic stem cells for therapeutic applications. Mol Ther. 2006 Jan;13(1):5-14.
- Heindryckx B, Leary TO, et al. Challenges and possible clinical applications of human embryonic stem cell research. Proceedings of the belgian royal academies of medicine. 2012.
- Dzobo K, Multipotent Human Mesenchymal Stem/Stromal Cells: An Updated Review on Historical Background, Recent Trends and Advances in their Clinical Applications. Preprints. 2021.
- Delsing L, Herland A, et al. Models of the blood-brain barrier using iPSC-derived cells. Mol Cell Neurosci. 2020. 107:103533.
- Alomari M, Almohazey D, et al. Role of Lipid Rafts in Hematopoietic Stem Cells Homing, Mobilization, Hibernation, and Differentiation. Cells. 2019. 8(6):630.
- Mitra A, Mishra L, et al. Technologies for deriving primary tumor cells for use in personalized cancer therapy. Trends Biotechnol. 2013. 31(6):347-54.