Circulating Tumor Cells as Cancer Biomarkers in the Clinic
Circulating tumor cells (CTCs) are cells that have shed into the vasculature or lymphatics from a primary tumor and are carried around the body in the circulation. In 1869, Tomas Ashworth observed CTCs in the blood of a man with metastatic cancer for the first time. CTCs, like a seed, can trigger another additional tumor at vital distant organs. The importance of CTCs in modern cancer research began in the mid-1990s. Now it has demonstrated that CTCs derive from clones in the primary tumor and is exist early on in the course of the disease.
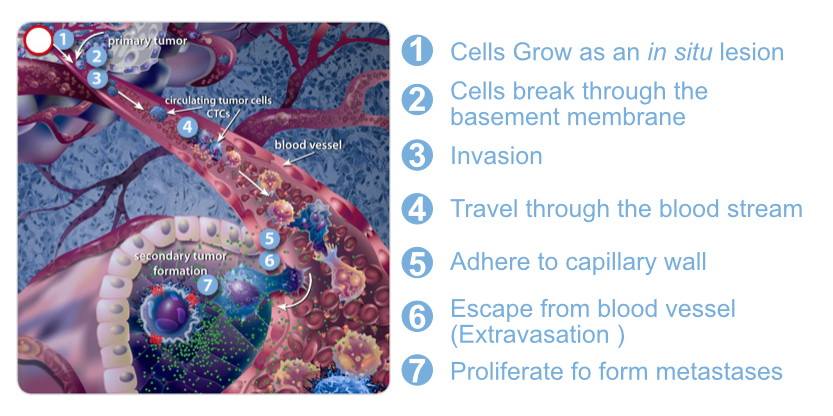
CTCs, as rare abnormal cells, comingled with billions of the normal red and white blood cells. The development of systems to detect CTCs has brought progress to cancer treatment. The molecular characterization of CTCs can aid in the development of new drugs, and their presence during treatment can help clinicians determine the prognosis of the patient. Their utility is in real-time monitoring of therapies, the identification of therapeutic and resistance targets, and understanding the process of metastasis. It also can be used to screen patients with family histories of cancer or with diseases that can lead to the development of cancer.
Here we will review some important aspects of CTCs, surveying the disease aspects where these cells have been investigated.
CTCs as Prognostic Factors in the Metastatic Setting
The development of enrichment systems and immunohistochemical detection of CTCs represents significant progress for the scientific community. The best known is the CellSearch® System, which separates the cells with magnetic beads coated with anti-epithelial cell adhesion molecule (EpCAM) antibody followed by flow cytometry of cells captured with anti-cytokeratin fluorescence.
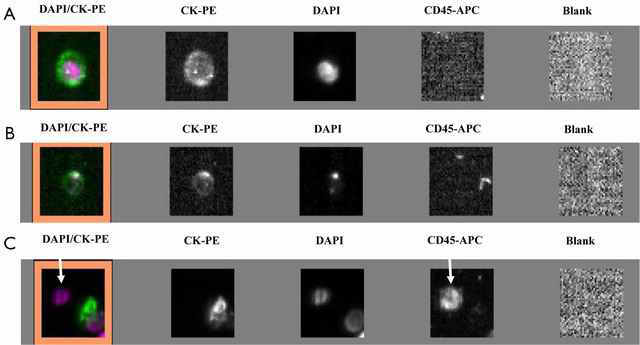 Figure 1. Circulating tumor cells detected in lung cancer patients using an indirect method, the CellSearch system. (A) Cell with a round morphology, a visible DAPI-positive nucleus, positive CK-PE staining in the cytoplasm and negative staining for CD45 is considered as typical intact CTC; (B) image of cell not included in the CTC count; (C) CD45-positive cell (arrows) is not considered as CTC. (Ilie, Marius, 2014)
Figure 1. Circulating tumor cells detected in lung cancer patients using an indirect method, the CellSearch system. (A) Cell with a round morphology, a visible DAPI-positive nucleus, positive CK-PE staining in the cytoplasm and negative staining for CD45 is considered as typical intact CTC; (B) image of cell not included in the CTC count; (C) CD45-positive cell (arrows) is not considered as CTC. (Ilie, Marius, 2014)
Using the CellSearch® System, Cristofanilli et al.(2004) reported a study of 177 patients with metastatic breast cancer, performing the CTC counts before and after the start of treatment for metastatic disease. It's suggested that patients with ≥5 CTCs/7.5 ml of blood when compared to those with less<5 CTCs/7.5 ml, had lower progression-free survival (2.7 versus 7 months, p <0.001) and reduced overall survival (10.1 versus 18 months, p <0.001). After the first segment following the beginning of treatment, this difference between the groups persisted (in relation to the survival and the number of CTCs). Furthermore, it was observed that about 70% of patients with metastatic disease had CTC counts above 1/7.5 ml of peripheral blood. This study provided key evidence for the use of CTCs.
CTCs as Prognostic Factors in Advanced Stages of Disease Studies

Studies have been done with non-metastatic cancer and the role of CTCs in prognosis also seems to be as important as it is in metastatic disease. In the study of Magni et al.(2014), 16 of 90 patients (19%) had CTCs ≥1 at the outset (t0) and a reduction in CTC number in cases of objective remissions. The proportion of patients with CTCs ≥1 decreased over time as the therapeutic course proceeded. Increasing CTC detection rate by enhancing the available laboratory tests and achieving better patient characterization would be productive.
Using immunofluorescence and immunohistochemistry techniques, Hong et al.(2016) isolated and identified CTCs in 100% of (29) patients with early (non-metastatic) breast cancer, indicating that this procedure allowed detection of these cells with greater accuracy, sensitivity, and specificity. In addition, they demonstrated in situ “naked eye” identification of the captured cancer cells via a simple colorimetric immunoassay.
The use of CTC in non-metastatic colorectal cancer requires very sensitive and specific detection methods. An international consensus on the assessment of detection method and markers needs to be finalized before incorporating CTC detection into risk stratification in the clinical setting.
CTCs as a Predictor of Drug Resistance
Advanced and metastatic solid tumors are commonly treated with chemotherapy, one of the most aggressive types of treatment. But resistance to chemotherapy is a very common issue in cancer, even with targeted therapies, resistance mechanisms, as well as toxic side effects, occur frequently.
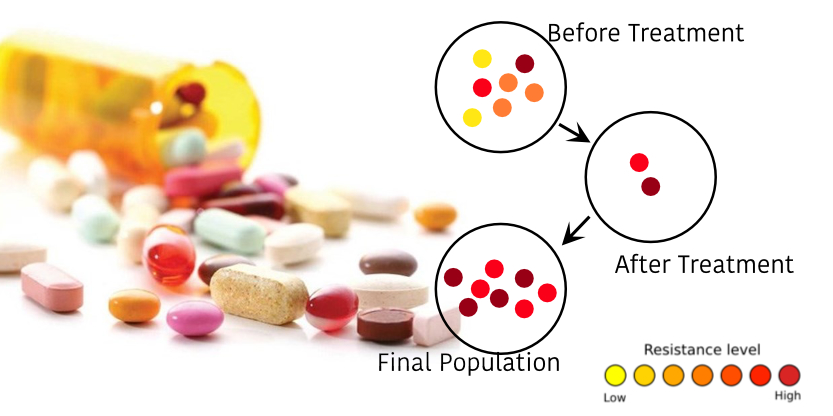
Many types of cancer cells have plasma membrane proteins that transport chemicals and toxins out of the cytoplasm. These proteins are mainly from the multi-drug resistance (MDR) family and have been widely studied. Studies on CTCs have been focused on their prognostic significance, but CTCs shed by both primary and metastatic cancers during tumor formation and progression are now considered to be a real-time "liquid biopsy" reflecting the disease complexity. There is no doubt that CTCs develop a new method for real-time monitoring of therapies, the identification of therapeutic and resistance targets, and understanding the process of metastasis.
CTCs have been demonstrated to be efficient markers for providing tumor information, presenting predictive markers, optimizing choices of therapeutic strategies, and thus opening new perspectives to achieve personalized medicine. Some MDR-related markers were successfully derived from CTCs, correlating with drug resistance (MRP1, MRP2, MRP4, MRP5, and MRP7).
CTCs as Prognostic Factors in Early Stages of Cancer
Wong et al.(2009) examined 101 patients with tumor, node, metastasis (TNM) stage I e III CRC, detecting CTC with a gastrointestinal-specific CK20. Sixty-two of 101 patients were followed for a period of 24 months and the association between preoperative elevated CK20 and recurrence was found to be highly significant (p < 0.001). The CTCs were an independent prognostic factor of survival (p < 0.005) in a multivariate regression analysis including TNM-stage, lymph node status, age, sex, tumor stage and degree of differentiation.
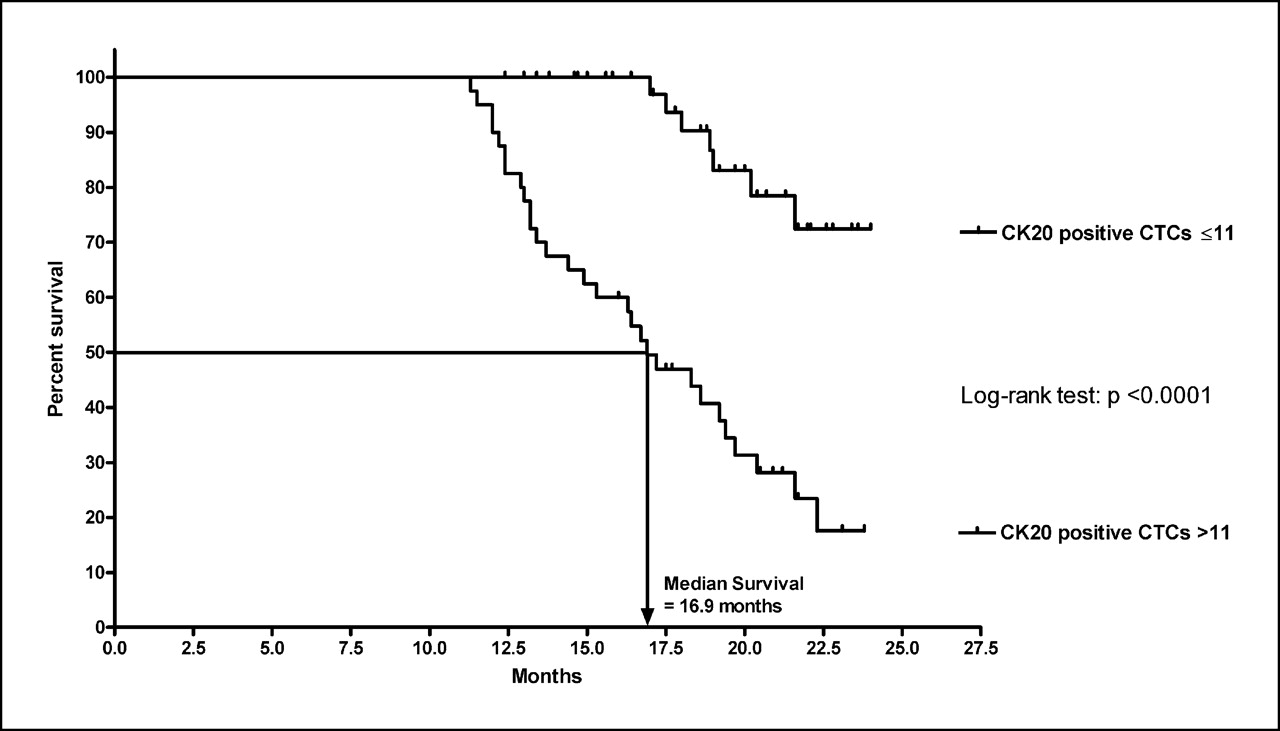 Figure 2. Kaplan-Meier overall survival curve for 43 colorectal cancer patients with CK20 pCTCs ≤11 and 40 colorectal cancer patients with CK20 pCTCs >11. (Wong, 2009)
Figure 2. Kaplan-Meier overall survival curve for 43 colorectal cancer patients with CK20 pCTCs ≤11 and 40 colorectal cancer patients with CK20 pCTCs >11. (Wong, 2009)
The main advantage of CTC analysis in early stages is based on the ease of obtaining a "liquid biopsy" and thus being able to monitor patients over the course of the disease, providing valuable information about the very early assessment of treatment effectiveness and helping towards establishing individualized therapies that will improve the efficiency with less cost and fewer side effects for cancer patients. More studies on the molecular characterization of CTCs in early stage may provide important information for the identification of therapeutic targets and understand resistance to therapies.
Prospect
There are investigators, who argue that the prognostic significance of CTC counts should not be ignored, even when the system used to evaluate CTCs-the CellSearch® System-has well-known limitations. CTC evaluations are still included as a biomarker in more than 400 clinical trials using various assays. We believe that CTCs studies have potential to help physicians use a more rational approach for management of both metastatic and non-metastatic tumors, reflecting solid tissue or mesenchymal cancers. However, we will need to develop a standard system and protocol in order to be able to use CTCs in routine clinical settings.
There are systems that provide for CTC isolation in a marker independent manner, by cytopathological analysis, which seems promising in capturing all malignant cells. Even considering their weak points, CTCs are one of the most promising and versatile biomarkers in translational oncology. As highlighted by Kang and Pantel (2013), viewing CTCs as a "liquid biopsy" opens new opportunities for genotyping and phenotyping micrometastatic cells derived from various distant sites, which, if adequately developed, may provide clinical oncology with more complete pictures of the evolution of cancers compared to those provided by biopsies of single metastatic sites.
Related Services
References
- llie, Marius.; et al. Current challenges for detection of circulating tumor cells and cell-free circulating nucleic acids, and their characterization in non-small cell lung carcinoma patients. What is the best blood substrate for personalized medicine?." Annals of translational medicine 2, no. 11 (2014).
- Cristofanilli.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004.351 (2004): 781-791.
- Magni E.; et al. Detection of circulating tumor cells in patients with locally advanced rectal cancer undergoing neoadjuvant therapy followed by curative surgery. Int J Color Dis. 2014, 29(9):1053–1059.
- Hong W.; et al. Multifunctional magnetic nanowires: a novel breakthrough for ultrasensitive detection and isolation of rare cancer cells from non-metastatic early breast cancer patients using small volumes of blood. Biomaterials. 2016, 106:78–86.
- Phillips KA.; et al. Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. JAMA. 2001, 286(18):2270–2279.
- Holohan C.; et al. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013, 13(10):714–726.
- Haber DA.; et al. The evolving war on cancer. Cell. 2011, 145(1):19–24.
- Wong, S.; et al. Clinical significance of cytokeratin 20-positive circulating tumor cells detected by a refined immunomagnetic enrichment assay in colorectal cancer patients. Clinical Cancer Research. 2009, 15(3), 1005-1012.
- Kang Y.; et al. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell. 2013, 23(5):573–581.