How to Choose the Right Antibody for Immunohistochemistry (IHC)
The selection of the right antibody is the most important decision in any immunohistochemistry experiment. This single reagent can make the difference between obtaining crisp, clean data that provides a direct answer to the biological question at hand and failing to obtain an interpretable signal or obtaining a misleading result, at best. When the wrong antibody is selected or when a good antibody is used improperly, IHC experiments can go astray. Problems that can ensue include non-specific staining, weak staining or staining that is not reproducible. In any of these situations, the time and resources put into the work are lost, and scientific conclusions can be in error. It is therefore essential to take a systematic and informed approach to antibody selection.
Understanding Antibody Basics
Antibodies are proteins that recognize specific epitopes on target antigens. They are Y-shaped molecules composed of variable regions that bind the antigen and constant regions that determine the class and host species.
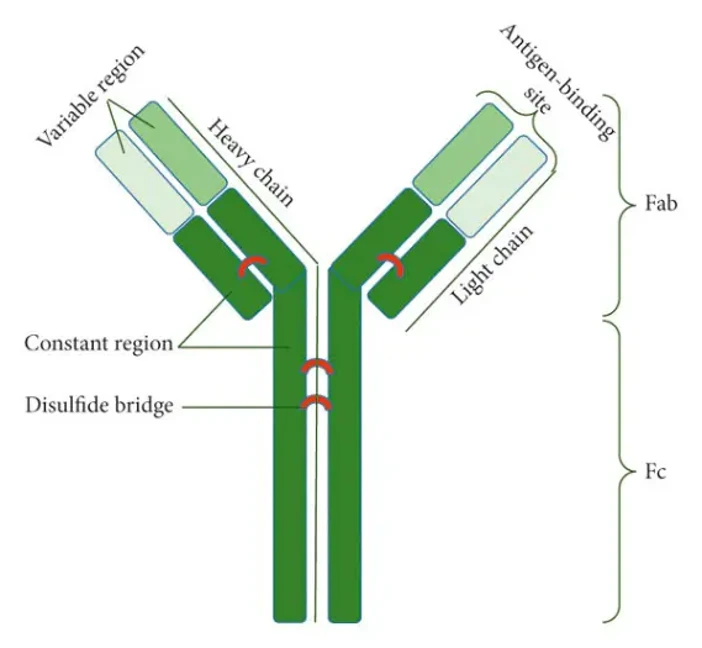
Primary vs. Secondary Antibodies
- Primary antibodies directly recognize the target protein.
- Secondary antibodies bind to primary antibodies and are often conjugated to a reporter, such as a fluorophore or enzyme, to enable visualization.
Monoclonal vs. Polyclonal Antibodies
- Monoclonal antibodies are produced by a single B-cell clone and recognize a single epitope. They are highly specific and very reproducible.
- Polyclonal antibodies are produced by multiple B-cell clones and recognize multiple epitopes. They usually provide a stronger signal, but with more background.
Understanding these distinctions helps researchers match the antibody to the experimental needs and the tissue type being studied.
Key Considerations for Choosing an IHC Antibody
Target Protein
- Protein Function and Localization: What is your target? Is it a surface receptor, cytoskeletal protein, nuclear transcription factor, secreted cytokine, etc. You will want to choose an antibody that is validated for the subcellular localization of your protein of interest. An antibody raised against a cytoplasmic domain may not recognize a nuclear isoform, and vice versa.
- Species Reactivity: Ensure that the antibody is explicitly validated to react with the protein of interest from the species used in your experiment (e.g., human, mouse, rat, etc.). An antibody raised against human EGFR will likely not cross-react with murine EGFR, for example, due to low sequence homology.
Antibody Specificity
- Monoclonal vs. Polyclonal: Monoclonal antibodies are typically more specific and are preferred when high specificity is required. Polyclonal antibodies can provide stronger signals but may have higher background staining. Consider the balance between specificity and signal strength based on your experimental needs.
- Validation and Characterization: Choose an antibody that has been validated specifically for IHC. Antibodies that have been tested by Western Blot, ELISA, and IHC will have the most detailed information on its specificity and sensitivity. Information such as images from IHC along with published citations can be invaluable for determining the right antibody for your application.
Antibody Affinity
- Affinity Constants: Antibody affinity (Kd) is defined as the strength of binding between a single antibody-binding site and a single epitope on the target antigen. Antibody affinities are expressed in dissociation constant (Kd) units, where a smaller Kd value correlates with a higher affinity. Higher affinity antibodies will bind more tightly and bind more efficiently, producing a stronger specific signal in a given experiment. These antibodies will also be more resistant to washing off during stringent wash steps.
- Cross-Reactivity: Scrutinize the product datasheet for information on potential cross-reactivity with unrelated proteins or homologous family members. Non-specific binding can mimic true positive signals and invalidate an experiment.
Detection Method
- Direct vs. Indirect Detection: Direct detection uses primary antibodies conjugated with reporters, while indirect detection uses labeled secondary antibodies to amplify the signal. The choice between these two methods is based on sensitivity needs and panel complexity.
- Fluorophore or Enzyme Conjugation: Depending on your detection system, choose antibodies conjugated with appropriate fluorophores for immunofluorescence or enzymes such as HRP for chromogenic IHC.
Tissue Fixation and Antigen Retrieval
- Fixation Methods: The choice of tissue fixation method (e.g., formalin, alcohol, etc.) can influence antigen preservation and antibody binding. Formalin fixation is the most common but can mask antigen epitopes. Ensure compatibility of the antibody with your fixation method.
- Antigen Retrieval Techniques: Antigen retrieval methods, like heat-induced epitope retrieval (HIER) or protease-induced epitope retrieval (PIER), can unmask epitopes obscured by fixation. Choose an antibody known to work well with your chosen antigen retrieval method.
A Stepwise Protocol for Antibody Selection
Follow this structured workflow to minimize uncertainty and maximize success.
- Define your Biological Target and Experimental Goal in as much detail as possible (What protein are you detecting? In what species? What is the question you want to answer? e.g. "Is phosphorylation of STAT3 increased in treated mouse liver sections?").
- Search for antibodies (use catalogs of reputable vendors as well as antibody search engines/databases to generate a list of candidates)
- Evaluate Validation Data for each candidate antibody (review provided IHC images carefully, confirm citations in PubMed for each experiment, and when possible, choose antibodies that have been validated using knockout/knockdown experiments)
- Pilot Test a few top candidates (purchase a small amount of your top 2-3 candidates and test them side-by-side on a well-characterized control tissue that you know is positive for your target, e.g. human tonsil for lymphoid markers)
- Concentration and Conditions (optimize concentration of the primary antibody by doing a chessboard titration to achieve the strongest specific signal with the least background noise. At the same time, optimize incubation times and antigen retrieval conditions)
- Controls and Documentation (begin with the right controls and document all protocol details, including the antibody lot number, from the start).
Troubleshooting Common Issues
Non-Specific Staining
Non-specific staining can occur due to high antibody concentration, inadequate blocking, or non-specific binding of the secondary antibody. To address this, dilute the antibodies appropriately, use blocking solutions to reduce non-specific binding, and select antibodies with high specificity.
Weak Signal
Weak signals may result from low antibody affinity, insufficient incubation time, or an incompatible detection method. To enhance the signal, choose antibodies with higher affinity, extend incubation times, or switch to a more sensitive detection method.
Background Staining
Background staining can be caused by non-specific binding of antibodies or incomplete washing. To minimize background staining, use highly specific antibodies, perform thorough washing steps, and optimize the blocking process.
In summary, the best approach to ensure excellent IHC results is to treat antibody selection as a hypothesis-driven, evidence-based process rather than an afterthought. By investing time in defining your target, carefully evaluating validation data, and following a systematic testing workflow, you can turn your IHC experiments from a high-risk/high-reward gamble to a reliable and powerful discovery tool.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
|---|---|
| Immunohistochemistry (IHC), Immunofluorescence (IF) Service | Creative Bioarray offers a comprehensive IHC service from project design, marker selection to image completion and data analysis. |
Reference
- Golpour M, Vatanpour P, et al. The Perspective of Therapeutic Antibody Marketing in Iran: Trend and Estimation by 2025. Adv Pharmacol Pharm Sci. 2021; 2021:5569590.