Skin Anti-Acne

Acne is a chronic inflammatory condition of the sebaceous unit of the hair follicle caused by androgen-induced increased sebum production, altered keratinization, inflammation, and bacterial colonization of the hair follicles on the face, neck, chest, and back by Propionibacterium Acnes.
Clinical features of acne include seborrhea, non-inflammatory lesions, inflammatory lesions, and scarring of various degrees. And the distribution of acne has been shown to correspond to the high density of follicular sebaceous gland units (face, neck, upper chest, shoulders, and back).
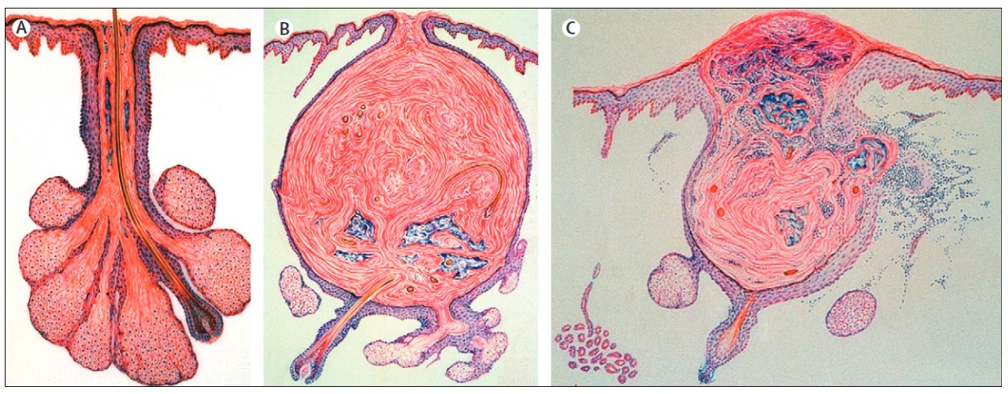 Figure 1. Normal sebaceous follicle (A) and comedo (B), and inflammatory acne lesion with rupture of follicular wall and secondary inflammation (C) (Williams, et al. 2012).
Figure 1. Normal sebaceous follicle (A) and comedo (B), and inflammatory acne lesion with rupture of follicular wall and secondary inflammation (C) (Williams, et al. 2012).
Creative Bioarray provides antimicrobial activity determination assay, in vitro skin model assays to evaluate the anti-acne efficacy of your products.
Your Needs
- To screen active ingredients/formulations, and characterize the efficacy or toxicity, or safety of any chemicals for acne
- To evaluate the anti-acne effect of potential cosmetic products
- To look for a CRO performing skin anti-acne efficacy testing
- To find a customized testing service related to acne
Our Capability
In vitro skin models available
- Human sebocytes
- Normal human epidermal keratinocytes (NHEK)
- Human follicle dermal papilla cells (HFDPC)
- Microorganisms (Propionibacterium acnes)
- Reconstructed human epidermis (RHE)
Efficacy and Screening Tests available
- Evaluating the anti-acne efficacy of active substances/compounds
- Evaluating the anti-acne effect of different formulations
- Evaluating inflammatory suppressive capacity
- Customized testing based on customer needs
Endpoints
- Cell viability
- Inflammatory factors/ mediators
- Bacteria inhibition rate
- Sebum excretion
- Keratinisation
- Histology (H&E, Nile red staining, histopathological scoring)
Techniques
- qPCR, qPCRarray, RT-PCR
- ELISA
- Western-Blotting
- Specific immuno-labeling
- Histology (H&E, etc.)
- Microbial inhibition assay
- RNA extraction
- Protein extraction
- Macroscopy
Study Examples
| Microorganism | Minimum inhibitory concentration (MIC) |
| Cutibacterium acnes | <1/2048 |
| P.acnes clinical isolate | <1/2048 |
| AE1, anti-acne extract 1; AE2, anti-acne extract 2 | |
Table 1. Minimum inhibitory levels of AE1 and AE2 on both isolates of Propionibacterium acnes (Kılıç, et al. 2019).
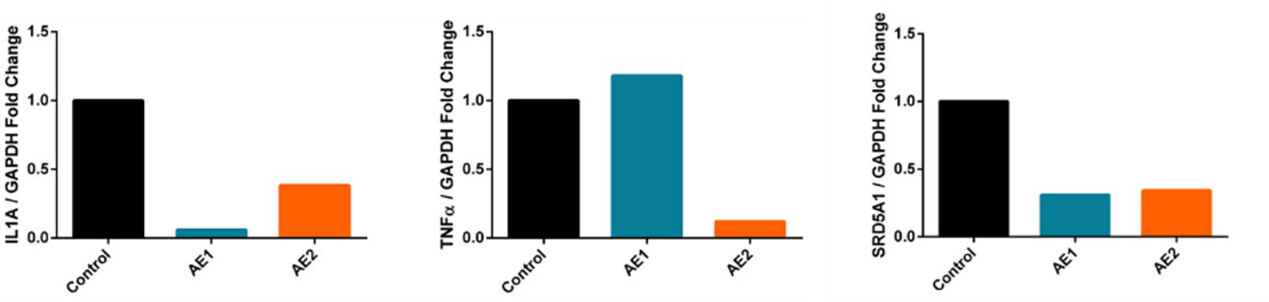 Figure 2. Expression levels of the selected genes as a fold change (Kılıç, et al. 2019).
Figure 2. Expression levels of the selected genes as a fold change (Kılıç, et al. 2019).
In vitro Skin Models
References
- Williams, Hywel C., Robert P. Dellavalle, and Sarah Garner. "Acne vulgaris." The Lancet 379.9813 (2012): 361-372.
- Kılıç, Songül, et al. "Efficacy of two plant extracts against acne vulgaris: initial results of microbiological tests and cell culture studies." Journal of cosmetic dermatology 18.4 (2019): 1061-1065.
Explore Other Options