What Are the Best Methods to Test Cardiotoxicity?
Detecting cardiotoxicity early in the drug development process is essential for mitigating late-stage failures and ensuring patient safety. Various methods, ranging from in vitro assays to in vivo models and clinical evaluations, have been developed to assess the cardiotoxic potential of drugs. Here, we provide an overview of the most commonly used methods for cardiotoxicity detection.
hERG channel screening
The hERG (human Ether-a-go-go Related Gene) channel is a well-established target for cardiotoxicity assessment due to its critical role in cardiac repolarization. Inhibition of hERG channels can lead to prolonged QT intervals and increased risk of arrhythmias. Automated patch clamp platforms, such as the QPatch and IonWorks systems, are widely used for high-throughput screening of hERG channel inhibition. These platforms offer rapid and accurate measurements of drug effects on hERG currents, allowing for the early identification of potential proarrhythmic risks.
Ion channel panels
In addition to hERG, other ion channels involved in cardiac electrophysiology, such as sodium (NaV1.5) and calcium (CaV1.2) channels, may also need to be assessed. Ion channel panels can be used to simultaneously evaluate the effects of drugs on multiple ion channels, providing a more comprehensive understanding of their potential electrophysiological impacts. These panels are often used in conjunction with hERG screening to better predict the overall cardiotoxic risk.
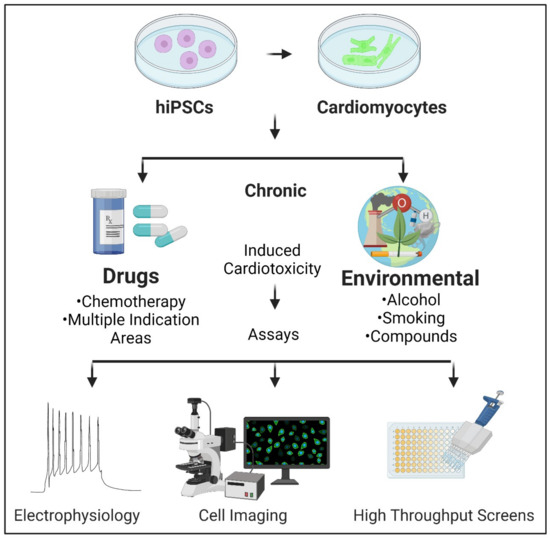
Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs)
Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) represent a more physiologically relevant model for studying cardiotoxicity. These cells can be differentiated from hiPSCs and cultured as 2D monolayers or 3D cardiac organoids. hiPSC-CMs can be used to assess various aspects of cardiotoxicity, such as changes in beating frequency, contractility, and intracellular calcium handling. Techniques such as multi-electrode arrays (MEAs) and impedance-based assays can be employed to monitor the electrical and mechanical activities of hiPSC-CMs in real-time, providing detailed insights into drug-induced cardiotoxic effects.
Mitochondrial function assays
Mitochondrial dysfunction is a common mechanism of cardiotoxicity that can lead to energy depletion and oxidative stress. Mitochondrial function assays, such as the Seahorse XF Analyzer, can measure oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) to assess mitochondrial health. These assays can help identify drugs that impair mitochondrial function and contribute to cardiotoxicity. Additionally, assays such as the Seahorse XF Analyzer can measure OCR and ECAR in live cells and tissues, providing insights into mitochondrial respiration and glycolytic activity.
Isolated heart perfusion (Langendorff System)
The Langendorff system is a classic ex vivo method for studying cardiac function. In this technique, isolated hearts are perfused with an oxygenated buffer solution, and various parameters of cardiac function can be measured, such as cardiac contractility, coronary flow, and electrophysiological properties. This method allows for the direct assessment of the effects of drugs on the heart without interference from systemic circulation and provides a controlled environment to study the acute effects of drugs on cardiac function under various physiological and pathological conditions.
Isolated cardiac muscle preparations
Isolated cardiac muscle preparations, such as papillary muscles or left ventricular strips, can be used to study the effects of drugs on myocardial contractility and relaxation. These preparations allow for the measurement of force development and length changes in response to drug exposure, providing detailed insights into drug-induced changes in cardiac mechanics. Techniques such as force transducers or video edge detection systems can be used to monitor contractile function in real-time.
Small animal models
Small animal models, such as mice and rats, are commonly used to study cardiotoxicity in vivo. These models allow for the assessment of the effects of drugs on cardiac function and structure over time and can be used to study the acute and chronic effects of drug candidates. Techniques such as echocardiography, telemetry, and histopathology can be used to evaluate changes in cardiac function, electrophysiology, and tissue morphology in these models. Transgenic mouse models, which express human cardiac ion channels or other relevant proteins, can provide more human-relevant data and help bridge the gap between in vitro and clinical studies.
Large animal models
Large animal models, such as dogs and pigs, offer a closer approximation to human cardiac physiology and are often used for more advanced preclinical studies. These models can be used to evaluate the chronic effects of drugs on cardiac function and structure and to study the pharmacokinetics and pharmacodynamics of drug candidates. Techniques such as hemodynamic monitoring, ECG telemetry, and cardiac MRI can be used to provide comprehensive assessments of cardiotoxicity in large animals.
Electrocardiography (ECG)
Electrocardiography (ECG) is a non-invasive method to monitor the electrical activity of the heart and can provide information about cardiac electrophysiology. It is commonly used in clinical trials to monitor changes in heart rate, rhythm, and QT intervals. Prolongation of the QT interval is a well-established marker of proarrhythmic risk, and drugs that cause significant QT prolongation can be flagged for further cardiotoxicity testing. Continuous ECG monitoring, such as Holter monitoring, can provide more detailed information about drug-induced changes in cardiac rhythm over extended periods.
Echocardiography
Echocardiography is a powerful tool for assessing cardiac function and structure. It can provide information about left ventricular ejection fraction (LVEF), fractional shortening, and wall motion abnormalities. Echocardiography can be used in clinical trials to monitor changes in cardiac function over time and to detect potential cardiotoxic effects of drugs. Advanced echocardiographic techniques, such as tissue Doppler imaging and strain rate imaging, can provide more detailed assessments of cardiac mechanics and may be able to detect early signs of dysfunction.
Biomarkers
Cardiac biomarkers, such as troponins, B-type natriuretic peptide (BNP), and creatine kinase-MB (CK-MB), can be released into the bloodstream in response to myocardial injury and stress. Elevated levels of these biomarkers in the blood can be indicative of cardiotoxicity. Regular monitoring of cardiac biomarkers in clinical trials can help to detect subclinical cardiotoxic effects and guide further cardiotoxicity testing.
Optical mapping
Optical mapping is a high-resolution technique for studying cardiac electrophysiology. It involves the use of voltage-sensitive dyes that can be loaded into cardiac cells to visualize the electrical activity of the heart in real-time. Optical mapping can provide detailed information about the mechanisms of arrhythmogenesis and the effects of drugs on cardiac electrical remodeling. This technique is particularly useful for studying complex arrhythmias and identifying potential proarrhythmic mechanisms.
Magnetic resonance imaging (MRI)
Cardiac MRI is a non-invasive imaging technique that uses magnetic fields and radio waves to generate detailed images of the heart. It can provide information about cardiac structure, function, and tissue properties. Cardiac MRI can be used to assess parameters such as LVEF, cardiac volumes, and wall thickness. It can also detect early signs of fibrosis and inflammation, which are common consequences of cardiotoxicity. Advanced MRI techniques, such as diffusion tensor imaging (DTI) and cine MRI, can provide even more detailed assessments of cardiac tissue properties and function.
Computational modeling
Computational modeling and in silico simulations are becoming increasingly popular for predicting cardiotoxicity. These models can integrate data from various sources, such as in vitro assays, animal studies, and clinical observations, to simulate the effects of drugs on cardiac function and electrophysiology. Computational models can help to identify potential cardiotoxic risks early in the drug development process and guide the design of more effective and safer drug candidates.
In conclusion, detecting cardiotoxicity requires a multifaceted approach that includes a combination of in vitro, ex vivo, in vivo, and clinical methods. Each method has its strengths and weaknesses, and their combined use can help to identify and mitigate cardiotoxic risks early in the drug development process. The use of advanced techniques, such as optical mapping, cardiac MRI, and computational modeling, will continue to improve our ability to predict and manage cardiotoxicity, leading to safer and more effective drug development.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
|---|---|
| In Vitro Cardiotoxicity | Through our comprehensive and detailed assessment methods, Creative Bioarray helps reduce the cardiac safety risks of your drugs, thereby minimizing the potential for development failure and associated economic losses. |
| hERG Safety Assay | Learn more about our hERG Safety Assay service for cardiotoxicity assessment. |
| QT Prolongation Assay | Creative Bioarray offers in vitro QT prolongation using human cardiomyocytes to evaluate the cardiotoxicity of compounds with QT interval as the indicator. |
| 3D Cardiovascular Toxicity Service | Creative-Bioarray offers the opportunity for highly predictable pre-clinical cardio-toxicity testing to help our customs to reduce both the cost and duration of bringing a new drug candidate to market |
Reference
- Narkar A, Willard JM, et al. Chronic Cardiotoxicity Assays Using Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPSC-CMs). International Journal of Molecular Sciences. 2022; 23(6):3199.