Cultivated Meat: What to Know?
Cultivated meat, sometimes referred to as lab-grown, clean, or cultured meat, is grown in a lab from a few animal cells. This production method eliminates the need to raise and farm animals for food. The resulting product is composed of the same cell types and arranged in a similar structure as conventional meat, thus replicating the sensory and nutritional properties of traditional meat.
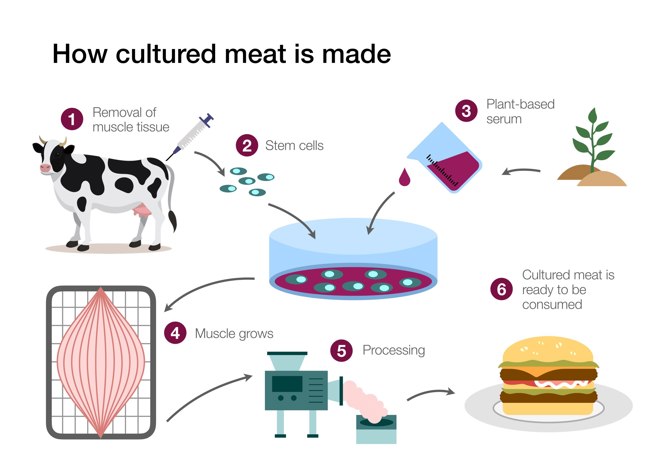
How is Cultivated Meat Produced?
Cultivated meat has the potential to replicate the taste, texture, smell, nutritional composition, and appearance of conventional meat. The production process can be divided into four steps:
-
A sample of stem cells is taken from a living animal. Stems cells are cells that can develop into other specialized types of cells in the body, such as blood, liver or muscle cells.
-
The stem cells are placed into large tanks called bioreactors, which contain culture media that recreate an environment similar to that of the cells in the animal's body and provide them with the nutrients they need to multiply.
-
By changing the culture media, the stem cells can be differentiated into the three main components of meat: muscle, fat, and connective tissue.
-
These cells are separated and arranged to "build" the type of meat being produced. This is called scaffolding. A scaffold is an edible material that supports the organization of meat cells into the desired shape, such as a steak or mincemeat. The scaffold does more than just hold the cells together. It also carries nutrients and helps them differentiate even further.
What are the Pros and Cons of Lab-Grown Meat?
Animal welfare is one of the reasons for producing lab-grown meat. This production method can produce meat without the need for raising and slaughtering large numbers of animals, so it has the potential to increase animal welfare within the food system. However, the process currently still relies on stem cells obtained from living animals, and the most widely used culture medium contains fetal bovine serum (FBS), which is collected from foetal blood at animal slaughterhouses, so it is not yet entirely slaughter-free.
Improved food safety as an advantage of lab-grown meat. This is because, in a controlled lab environment, meat products will not face the traditional risk of contamination by pathogenic bacteria such as E. coli and Campylobacter, which usually come from the gut of livestock. However, as with any industrial food production, microbiological or chemical contamination can still occur at different stages.
More sustainable protein production is essential in the next 30 years. However, it is not yet clear whether mass-scale production of meat in the lab is more environmentally sustainable than traditional animal husbandry. Although fewer resources such as water, land and fertilizers are needed than growing feed crops and livestock, other requirements are more complicated. For example: While a reduction of cattle farming would decrease methane emissions that contribute to climate change, lab-based meat production could generate problematic levels of carbon dioxide emissions over the long term.
Trends in the Development and Utilization of Cultivated Meat Cell Lines
The cell lines used for cultivated meat production ultimately determine many downstream variables that need to be considered. As starting material, cells that can self-renew and differentiate into the cell types that make up meat tissue are the most attractive. Generally, there are three viable cell options, including embryonic stem cells (ESCs), muscle satellite cells, and induced pluripotent stem cells (iPSCs), which can serve as sources of cells for cultivated meat. Muscle satellite cells have been selected as the preferred cell source for skeletal muscle tissue engineering. Once sufficient muscle satellite cells are prepared, they are induced to differentiate into myotubes or muscle fibers. However, it is difficult to maintain their continuous replication in vitro. ESCs are isolated from the early stages of embryonic development that can proliferate indefinitely and differentiate into muscle after stimulation in vitro. So far, no successful experiments have proven that they can be used to make cultivated meat. iPSCs are similar to ESCs in that they can differentiate into a variety of cell types under the action of specific signal molecules. The fact that porcine iPSCs can efficiently generate skeletal muscle in vitro provides an approach to other pluripotent cell lines. But some issues need to be clarified before use: 1) how to improve the proliferation ability of iPSCs; 2) develop methods to guide iPSCs to differentiate; 3) dedifferentiate and transdifferentiate into muscle satellite cells.
Accelerate your programs by leveraging Creative Bioarray's extensive inventory and prospective network of cell products.
| Cat. No. | Product Name |
|---|---|
| CSC-C0523Z | Porcine Skeletal Muscle cells |
| CSC-C0521Z | Porcine Skeletal Muscle Satellite cells |
| CSC-C00423L | Porcine Bone Marrow Mesenchymal Stem Cells |
| CSC-I2067Z | Immortalized Porcine Skeletal Muscle Satellite Cells |
| CSC-00860L | Chicken iPS Cell Line |
| CSC-C0525Z | Chicken Skeletal Muscle Cells |
| CSC-I9234L | Immortalized Chicken Skeletal Muscle Cells |
| CSC-I2225Z | Immortalized Chicken Adipose Cells |
| CSC-C0524Z | Bovine Skeletal Muscle Cells |
| CSC-I2302Z | Immortalized Bovine Skeletal Muscle Myoblasts |