Plasma Stability Assay
- Service Details
- Features
- FAQ
- Explore Other Options
Plasma stability is often one of the primary determinants of a compound's systemic exposure, efficacy and safety. Following administration, a compound encounters numerous plasma enzymes and reactive components capable of rapidly cleaving ester, amide, or peptide bonds. Such hydrolysis can reduce the circulating concentration of the compound and cause poor pharmacokinetics (PK) or loss of pharmacological activity.
Early assessment of plasma stability in drug discovery can provide valuable insight into metabolic liability, helping to distinguish whether instability originates from plasma hydrolysis or hepatic metabolism. Insufficient plasma stability often leads to failure to achieve desired bioavailability and excessive stability can lead to accumulation and/or prolonged exposure for some analogs.
Our Plasma Stability Assay Service
We offer a scalable plasma stability testing service with flexible throughput — from single-compound bespoke studies to high-throughput screening panels across multiple species. Data are generated by quantitative LC-MS/MS and reported as percent remaining over time and calculated in vitro half-life (t½) under defined conditions.
Workflow
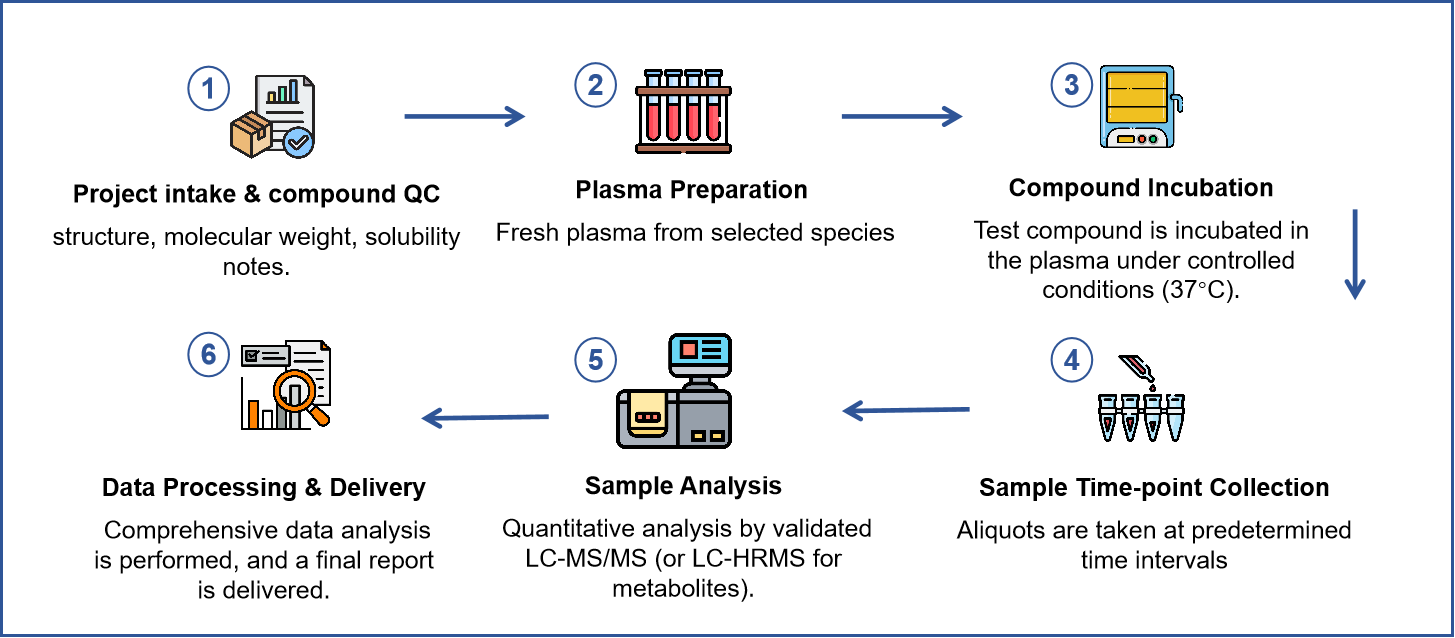
Protocol
| Parameter | Details |
| Species | Mouse, Rat, Dog, Cynomolgus Monkey, Human |
| Test Compound Concentration | 1 μM (different concentrations available) |
| Plasma Concentration | 100% (Standard, undiluted plasma) |
| Final Solvent Concentration | < 1% DMSO |
| Incubation Time Points | 0, 5, 15, 30, 60, 120 minutes |
| Incubation temp | 37°C |
| Compound Requirements | ≥ 1 mg dry compound or 50 µL of 10-20 mM stock DMSO solution |
| Positive Control | A compound with known, rapid plasma clearance (e.g., verapamil, propantheline, or internally selected standards) |
| Analytical Method | UPLC-MS/MS (quantitative), LC-HRMS for metabolite ID if required. |
| Data Delivery | Half-life (t1/2), Percent Remaining at each time point, Quality Control metrics. |
Explore Our More Stability Testing Services:
Quickly assesses the metabolic activity of compounds in the hepatic cytosol without requiring a complete cell structure, suitable for high-throughput screening of early compound libraries.
Analyzes the metabolic rate of compounds in liver microsomes, predicts in vivo clearance (CL), and supports cross-species difference analysis.
Conduct metabolic studies using complete human hepatocyte models to provide cellular-level metabolic information, aiding in the prediction of a drug's clearance rate in vivo.
Why Choose Us
Flexible Compound Handling
From highly lipophilic to poorly soluble molecules — our optimized protocols minimize non-specific binding and deliver precise free-drug quantification.
High-Throughput Efficiency
Automated liquid handling and high-speed LC-MS/MS systems support both rapid screening and accurate assays for every development stage.
Fast & Reliable Turnaround
Optimized workflows and streamlined data analysis mean you get trusted results — right when you need them.
Fully Integrated Solutions
Seamlessly combine with our microsomal and hepatocyte stability assays for a complete ADMET and metabolic stability profile.
FAQ
What does the half-life (T1/2) result tell me about my compound?
The half-life will provide the time it takes to reach 50% degradation of the parent compound. A short half-life (e.g., <30 minutes) indicates high metabolic instability of a compound in plasma, potentially requiring a chemical modification (prodrug approach) or resulting in poor exposure. On the other hand, a long half-life is usually considered a good property for less frequent dosing.
Which species should I test?
We recommend running human and the key nonclinical species that you intend to use for PK/PD (usually mouse, rat, cynomolgus). Testing human plasma at an early stage is important to identify any potential liabilities unique to humans. Running the test in parallel can also highlight any significant species differences which can be informative for species selection.
What is the difference between plasma stability and liver microsomal stability?
Plasma stability assesses degradation by enzymes present in the blood (e.g., esterases, amidases), while liver microsomal stability focuses primarily on hepatic Phase I metabolism (e.g., CYP450 enzymes). A compound can be stable in one system but not the other. Assessing both provides a complete picture of metabolic liability.
Can you determine the metabolites formed during the incubation?
The standard Plasma Stability Assay reports on the disappearance of the parent compound only. However, as a separate service, we provide a Metabolite Identification Service using high-resolution MS (HR-MS) to accurately identify and characterize degradation products.
Explore Other Options