EAE Models
Experimental autoimmune encephalomyelitis (EAE) is the most widely accepted model that recapitulates typical pathological hallmarks of multiple sclerosis (MS), which is characterized by T-cell and monocyte infiltration in the central nervous system (CNS). EAE induced mouse model was first introduced by Olitsky et al. in 1949. And over the past 60 years, lab mice have gradually become the most powerful EAE model in part due to the extensive availability of multiple transgenic and genetic editing approaches in mice for extensive mechanistic studies. Additionally, EAE induced rat model is used to study pathological mechanism of MS as well.
Creative Bioarray specializes in providing customized pharmacodynamic research services to help customers assess the efficacy of drug candidates and study the associated pathological mechanisms through EAE models.
EAE models include but not limited to:
- Myelin Oligodendrocyte Glycoprotein (MOG)-Induced Experimental Autoimmune Encephalomyelitis (EAE) Model
- Proteolipid Protein (PLP)-Induced Experimental Autoimmune Encephalomyelitis (EAE) Model
- Myelin Basic Protein (MBP)-Induced Experimental Autoimmune Encephalomyelitis (EAE) Model
Our capabilities
- We utilize EAE Models to test therapeutic candidates targeting MS.
- We assess the severity of MS through EAE clinical score system.
- We quantitatively assess lymphocyte infiltration, demyelination and axonal damage by Histopathological techniques.
- We use SPECT/CT to detect inflammation in the brain and spinal cord.
Assays available
- Histopathological evaluation
- SPECT/CT monitoring
- Flow cytometry
With extensive experience in the field of MS, we are confident to help you to overcome any upcoming challenges. Our experts are fully capable of customizing our protocols and assays to meet your specific needs. With our help, we wish to facilitate your research with high efficiency.
Study examples
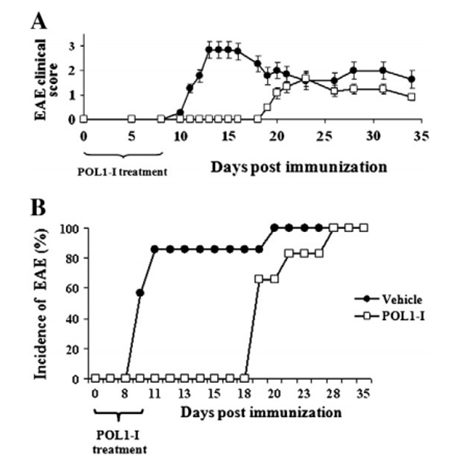 Figure. 1. POL1-I suppression of EAE.Chronic-progressive EAE was induced in C57BL/6J mice by subcutaneous immunization with MOG35–55 peptide, Mycobacterium Tuberculosis H37Ra and incomplete Freund's adjuvant. Pertussis toxin was injected intraperitoneally on the day of immunization (Day 0) and repeated 2 days later. POL1-I was administrated from the day of immunization until EAE onset (score = 1.0) in at least 30% of the animals of the Vehicle group. Mice were monitored daily for clinical signs of EAE and delay in the time of EAE onset and decreased disease severity in POL1-I treated mice are demonstrated. A. Daily mean clinical EAE scores of each group. B. Percentage incidence of EAE in each group plotted against time. White squares represent POL1-I group, black dots represent Vehicle group.
Figure. 1. POL1-I suppression of EAE.Chronic-progressive EAE was induced in C57BL/6J mice by subcutaneous immunization with MOG35–55 peptide, Mycobacterium Tuberculosis H37Ra and incomplete Freund's adjuvant. Pertussis toxin was injected intraperitoneally on the day of immunization (Day 0) and repeated 2 days later. POL1-I was administrated from the day of immunization until EAE onset (score = 1.0) in at least 30% of the animals of the Vehicle group. Mice were monitored daily for clinical signs of EAE and delay in the time of EAE onset and decreased disease severity in POL1-I treated mice are demonstrated. A. Daily mean clinical EAE scores of each group. B. Percentage incidence of EAE in each group plotted against time. White squares represent POL1-I group, black dots represent Vehicle group.
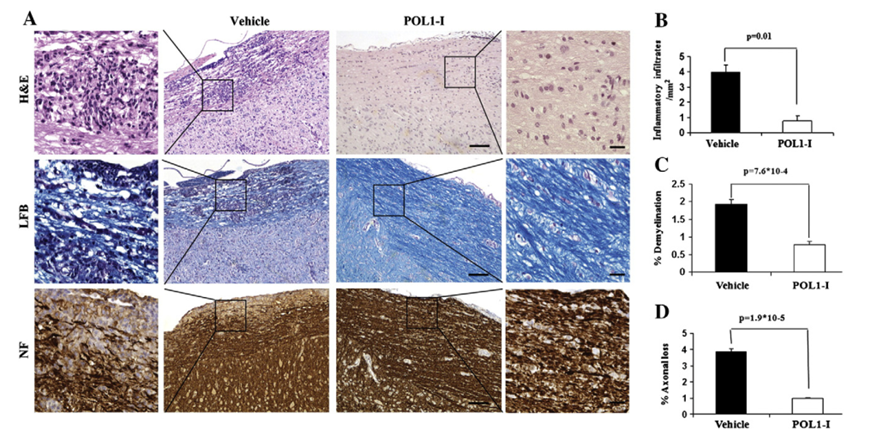 Figure. 2. Attenuation of EAE by POL1-I. A. Representative images of H&E staining (upper panel), LFB (middle panel) and NF staining (lower panel) in adjacent longitudinal spinal cord sections, day 14 post-POL1-I treatment. The boxed areas in the middle images are ×10 magnification. The squared external images are ×40 magnified images representative of solid squares in corresponding×10 images and point to areas of cellular infiltration, demyelination and axonal loss in the vehicle mice sections compared to POL1-I sections. Scale bar in middle images = 100 μm, magnified boxed images = 20 μm. B. Mean number of inflammatory infiltrates per mm2 in adjacent longitudinal spinal cord sections. C. The percentage of demyelinating areas in adjacent longitudinal spinal cord sections. D. The percentage of axonal loss area in adjacent longitudinal spinal cord sections. White columns represent POL1-I treated group and black columns represent Vehicle group.
Figure. 2. Attenuation of EAE by POL1-I. A. Representative images of H&E staining (upper panel), LFB (middle panel) and NF staining (lower panel) in adjacent longitudinal spinal cord sections, day 14 post-POL1-I treatment. The boxed areas in the middle images are ×10 magnification. The squared external images are ×40 magnified images representative of solid squares in corresponding×10 images and point to areas of cellular infiltration, demyelination and axonal loss in the vehicle mice sections compared to POL1-I sections. Scale bar in middle images = 100 μm, magnified boxed images = 20 μm. B. Mean number of inflammatory infiltrates per mm2 in adjacent longitudinal spinal cord sections. C. The percentage of demyelinating areas in adjacent longitudinal spinal cord sections. D. The percentage of axonal loss area in adjacent longitudinal spinal cord sections. White columns represent POL1-I treated group and black columns represent Vehicle group.
Quotation and ordering
If you have any special needs or questions regarding our services, please feel free to contact us. We look forward to cooperating with you in the future.
Reference
Achiron A, et al. Polymerase I pathway inhibitor ameliorates experimental autoimmune encephalomyelitis[J]. Journal of Neuroimmunology, 2013, 263(1-2):91-97.