CHO Cell Line Development
Biological therapeutics and diagnostics (theragnostic) are rapidly growing products in the pharmaceutical market. They include various monoclonal antibodies (mAbs), vaccines, hormones, and other proteins, all of which have a wide range of applications. A significant portion of available biologics are recombinant proteins, most of which are produced in mammalian expression platforms.
Mammalian expression-based systems involve various cell lines of different origins, from hamster (CHO, BHK), human (HEK293, HT-1080, PER.C6, CAP, HKB-11, Huh-7), and mouse (NS0, Sp2/0). However, 70% of biologics, and almost all mAbs, are produced in Chinese hamster ovary (CHO) cells, as the most commonly used and preferred hosts for biopharmaceutical protein production.
CHO Expression Platform and Its Advantages
- CHO cells are mammalian cells, providing a closer resemblance to human cells compared to other expression systems such as bacterial or yeast cells. This similarity ensures proper post-translational modifications and protein folding, leading to the production of functional and stable biotherapeutics.
- CHO cells also exhibit a high growth rate, allowing for large-scale production of proteins. They can be easily adapted to suspension culture, which simplifies the upstream processing and facilitates scalability. Additionally, CHO cells have a robust host cell machinery and can handle complex and challenging proteins, including monoclonal antibodies, enzymes, and cytokines.
- CHO ori cell line was isolated and immortalized by Theodore Puck in 1956. Despite sharing the same common ancestor, different CHO lineages exhibit substantial genetic heterogeneity as a result of extensive mutagenesis and clonal selection. These genetic differences, which affect karyogram and epigenetic variations, also, account for host cell-specific differences among the most widely used CHO cell lines (mostly CHO-K1, CHO DXB11, CHO-S, and CHO DG44).
CHO Cell Line Development Process
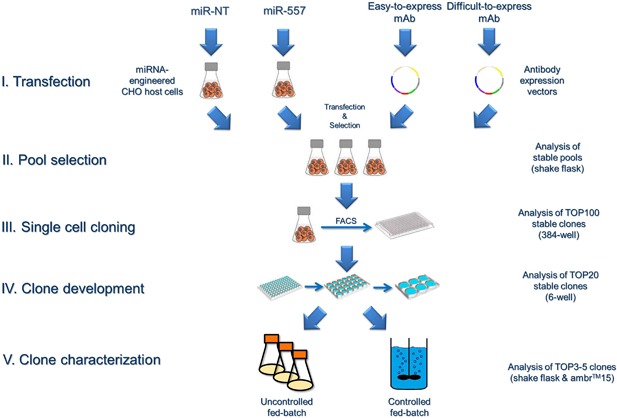 Fig. 1 Overview of the cell line development process. (Fischer S, et al., 2017)
Fig. 1 Overview of the cell line development process. (Fischer S, et al., 2017)
- Cell line isolation. CHO cells with desirable characteristics, such as high growth rate and protein expression capacity, are isolated from a parental CHO cell culture or obtained from a commercial source.
- Clone selection. Single CHO cells are isolated and cultured individually in a process known as clonal selection. This is done to ensure that each cell line derived from a single cell has similar characteristics and productivity.
- Expression vector construction. A DNA construct, known as an expression vector, is created to carry the gene of interest that codes for the protein to be produced. The expression vector is designed to ensure high expression levels in CHO cells and to enable stable integration of the gene into the cell's genome.
- Transfection. The CHO cells are transfected with the expression vector using techniques such as chemical transfection or electroporation. Transfection allows the introduction of the gene into the CHO cells' genetic material.
- Selection and Screening. After transfection, the CHO cells are subjected to a selection process to identify cells that have successfully integrated the gene of interest. This is often done by incorporating a selectable marker gene, such as resistance to a specific antibiotic, into the expression vector. Only cells that have taken up the selectable marker gene and the expression vector can survive in the presence of the selecting agent. Screening techniques, such as fluorescent markers or enzyme assays, may also be used to identify high-producing cell lines.
- Clone expansion and characterization. The selected CHO cell clones are expanded and characterized for their growth and productivity. This includes assessing their growth rate, protein expression levels, protein quality, and stability over time.
CHO Cell Line Optimization
- After establishing a stable CHO cell line, optimization strategies are employed to enhance protein production yield and quality.
- Media optimization plays a critical role in maximizing protein expression. The composition of the culture media is tailored to provide the necessary nutrients, growth factors, and supplements to support high cell density and efficient protein production.
- Process optimization involves optimizing cultivation conditions such as pH, temperature, dissolved oxygen, and agitation speed, to promote cell growth and productivity. Additionally, the optimization of feeding strategies and the implementation of continuous or fed-batch culture systems can further enhance protein production.
- Moreover, genetic engineering techniques are employed to improve protein expression levels. Manipulation of the CHO cell genome can be achieved through gene amplification, promoter optimization, or the introduction of specific genetic elements to enhance protein production and stability.
Creative Bioarray Relevant Recommendations
CHO cell line development is a complex and intricate process that requires expertise in cell biology, genetic engineering, and bioprocess optimization. Creative Bioarray excels in this domain, providing comprehensive services and high-quality products, such as CHO-SSR1 and CHO-SSR2, to enable the efficient production of biotherapeutic proteins.
Reference
- Fischer S, et al. (2017). "miRNA engineering of CHO cells facilitates the production of difficult-to-express proteins and increases success in cell line development." Biotechnol Bioeng. 114 (7), 1495-1510.