Parallel Artificial Membrane Permeability Assay (PAMPA)
- Service Details
- Features
- FAQ
The evaluation of potential pharmaceutical candidates at early development stages depends heavily on understanding drug absorption. The Parallel Artificial Membrane Permeability Assay (PAMPA) functions as a cost-efficient in vitro model that enables researchers to conduct high-throughput assessments of drugs' passive transport properties. Through a lipid-infused artificial membrane, PAMPA measures the permeability from a donor to an acceptor compartment, providing a rapid screening alternative that bypasses the complexities of cell-based assays.
By leveraging a sophisticated lipid-infused artificial membrane setup, our PAMPA services simulate critical biological barriers such as the gastrointestinal tract and blood-brain barrier. By facilitating rapid and economical evaluations, PAMPA plays a critical role in bringing effective pharmaceuticals to market efficiently.
Why PAMPA?
- Simplicity and Cost-effectiveness: PAMPA's straightforward methodology delivers results at a fraction of the cost of cellular assays, eliminating the need for extensive cell culture maintenance.
- Versatile Application: Capable of assessing permeability across a wide pH range, PAMPA aids in the early understanding of how oral compounds are absorbed throughout the gastrointestinal tract.
- Passive Diffusion Focus: The assay concentrates solely on passive transcellular permeation, avoiding confounders associated with active transport mechanisms.
- High-throughput Capacity: PAMPA can be automated using 96-well plates, allowing the processing of thousands of samples per day.
- Good Reproducibility: The PAMPA model has good reproducibility, ensuring the stability and reliability of experimental results.
Our PAMPA Methods
GIT-PAMPA: It is used to evaluate the permeability of drugs in the gastrointestinal tract and simulate the intestinal environment.
BBB-PAMPA: This method assesses the permeability of compounds across the blood-brain barrier.
Skin-PAMPA: Specializes in evaluating the permeation of compounds through the skin barrier.
Workflow
1
Membrane Preparation
Coat each well of a 96-well microtiter filter plate with a lipid membrane.
2
Assembly
Position the donor plate onto a pre-filled acceptor plate with buffer solution, forming a "sandwich".
3
Incubation
Maintain the assembly under constant shaking at room temperature to facilitate diffusion.
4
Disassembly and Analysis
Separate the components and measure drug concentration in both compartments using UV spectrophotometry or LC-MS/MS for permeability calculation.
5
Data Evaluation
Each compound's permeability is assessed in multiple replicates for accuracy.
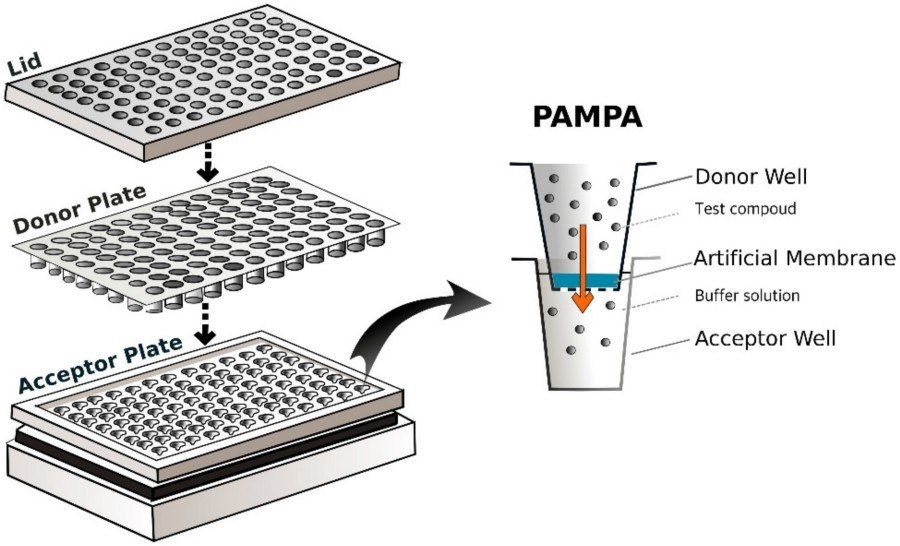 Fig. 1. Parallel Artificial Membrane Permeability Assay (PAMPA) 96-well setup (left); a single well of
PAMPA (right) (Ponmozhi J, Dhinakaran S, et al., 2021).
Fig. 1. Parallel Artificial Membrane Permeability Assay (PAMPA) 96-well setup (left); a single well of
PAMPA (right) (Ponmozhi J, Dhinakaran S, et al., 2021).
| PAMPA | Details |
| Test Article Concentration | 10 μM |
| Number of Replicates | 3 |
| Membrane Composition | Tri-layers of Phospholipids (Pre-coated) |
| Incubation Time | 4 hours |
| Test Article Requirements | 50 µL of 10 mM DMSO solution Brutto formulas are required. |
| Analysis Method | LC-MS/MS quantification |
| Data Delivery | Permeability (x10-6 cm/s). Full study report is provided. |
| Positive Control | Propranolol (high permeability) |
| Negative Control | Atenolol (low permeability) |
Features
Expertise and Reliability
With extensive experience, our team ensures reliable and precise data reporting to support your drug development pipeline.
Comprehensive Support
We offer full technical support and consultation services to optimize your PAMPA assay needs.

Tailored Solutions
Customizable assay conditions and analytics are available to meet specific client requirements, enhancing research flexibility.
FAQ
Q1: How should PAMPA data be interpreted?
PAMPA evaluates drug permeability through artificial membranes by analyzing passive diffusion which serves as the main route through which drugs are absorbed in the gastrointestinal tract as well as across the blood-brain barrier and cellular membranes. The classification of compounds into either low permeability (Pe < 1.5 x 10-6 cm/s) or high permeability (Pe > 1.5 x 10-6 cm/s) assists researchers in selecting potential candidates for additional development processes.
Q2: Is PAMPA applicable to all types of compounds?
PAMPA provides excellent evaluation of passive transport attributes but lacks the ability to measure active transport processes. To obtain complete absorption data for compounds, researchers should combine PAMPA testing with cell-based methods such as Caco-2 assays.
Q3: What are the differences between PAMPA and other permeability testing methods?
The primary distinctions between PAMPA and permeability testing methods such as Caco-2 and MDCK cell models lie in the type of model they use and their operational complexity.
- Cell-free model: PAMPA employs synthetic membranes in its cell-free design while Caco-2 and MDCK models operate using live cells. The use of PAMPA removes any influence of cellular differences and metabolic processes when measuring permeability.
- Simplified operation: PAMPA provides a simpler operation compared to cell-based models because it eliminates the need for cell culture which streamlines experimentation and decreases time requirements.
- High-throughput screening: PAMPA's simplified operational process makes it exceptionally suitable for high-throughput screening which allows rapid permeability evaluation of compounds during early drug development phases.
- Cost-effectiveness: PAMPA provides a more cost-effective permeability testing option than cell models such as Caco-2 because it eliminates the need for cell culture.
- Data comparability: Caco-2 and MDCK models potentially deliver data that better represents physiological conditions for complex transmembrane permeability characterization because they reproduce biological barriers more accurately than PAMPA which provides useful permeability data.
Quotation and ordering
If you have further inquiries or need additional assistance, please contact our expert team.
Reference
- Ponmozhi J, Dhinakaran S, et al. Development of Skin-On-A-Chip Platforms for Different Utilizations: Factors to Be Considered. Micromachines. 2021; 12(3):294.
Explore Other Options