Gallbladder Tumor Cells
- Background
- Applications
- Scientific Data
- FAQ
The gallbladder, a small pear-shaped organ located just beneath the liver, is responsible for storing and concentrating bile, a crucial fluid that aids in the digestion of fats. However, in some individuals, abnormal cell growth can occur within the gallbladder, leading to the formation of tumors. These tumors can vary in their characteristics, ranging from benign (non-cancerous) to malignant (cancerous), each with its own set of implications for the patient's health and well-being.
Molecular Characteristics of Gallbladder Tumor Cells
- Genetic mutations and alterations. Mutations in the TP53 gene are commonly observed in gallbladder tumor cells, contributing to uncontrolled cell proliferation, evasion of apoptosis, and tumor progression. Activating mutations in the KRAS gene are prevalent in gallbladder cancer, driving aberrant signaling pathways involved in cell survival, proliferation, and invasion. Mutations in the PIK3CA gene lead to dysregulated PI3K/AKT/mTOR signaling cascades in gallbladder tumor cells, promoting cell growth and survival. Targeting these mutations and alterations represents a potential therapeutic strategy.
- Epigenetic alterations. Aberrant DNA methylation patterns play a crucial role in gallbladder cancer pathogenesis, silencing tumor suppressor genes and activating oncogenes. Alterations in histone modifications influence gene expression patterns in gallbladder tumor cells, regulating chromatin structure and transcriptional activity. Altogether, epigenetic modifications contribute to tumor heterogeneity and may serve as diagnostic and prognostic markers.
- Signaling pathway dysregulation. Dysregulated Wnt/β-catenin signaling is frequently observed in gallbladder cancer, promoting cell migration, invasion, and epithelial-mesenchymal transition. Inhibiting aberrant Wnt pathway activation may impede tumor progression. Aberrant Notch signaling in gallbladder tumor cells influences cell fate decisions, proliferation, and apoptosis, contributing to tumor growth and metastasis. Targeting Notch signaling components presents a promising avenue for therapeutic intervention.
Cancer research and drug development
Gallbladder tumor cells have become an invaluable tool in the field of cancer research, serving as a model system to explore the genetic and molecular mechanisms that drive the initiation and progression of this disease. By studying the behavior and characteristics of these cells, researchers have been able to identify novel therapeutic targets and develop innovative drug candidates that hold promise in the treatment of not only gallbladder cancer but also other types of cancers.
Tissue engineering and regenerative medicine
By harnessing the proliferative and adaptable nature of gallbladder tumor cells, researchers have been able to create complex tissue structures that mimic the characteristics of the native gallbladder. These models can be employed for drug testing, toxicology studies, and the development of personalized therapeutic strategies, ultimately accelerating the translation of novel treatments from the laboratory to the clinic.
Furthermore, scientists have also investigated the potential of gallbladder tumor cells in the field of regenerative medicine. By understanding the signaling pathways and cellular mechanisms that drive the growth and differentiation of these cells, researchers aim to explore their ability to contribute to the repair and regeneration of damaged or diseased tissues, opening up new avenues for the treatment of various medical conditions.
PLEK2 Promotes Migration, Invasion, and Metastasis of GBC Cells
Gallbladder cancer (GBC) is an extremely malignant tumor with a high mortality rate. Little is known about its invasion and metastasis mechanism so far. To investigate the causal role of PLEK2 in GBC progression, PLEK2 down-regulation NOZ and GBC-SD cells (NOZ-shPLEK2, GBC-SD-shPLEK2, respectively), as PLEK2 overexpression NOZ and GBC-SD cells (NOZ-PLEK2, GBC-SD-PLEK2, respectively) were constructed.
Cell proliferation assay showed no difference between PLEK2 knockdown and control cells. Meanwhile, the transwell migration assay indicated that PLEK2 knockdown or overexpression significantly inhibited or promoted cell migration in corresponding GBC cells, respectively. Similar to the migration assay, the transwell invasion assay also showed the same results (Fig. 1a, c). Therefore, these in vitro studies indicated that PLEK2 promoted GBC cell migration and invasion. In addition, whether PLEK2 could promote GBC tumor metastasis in xenograft models was investigated. In vivo studies showed that PLEK2 knockdown exhibited fewer liver metastatic foci whereas PLEK2 overexpression displayed more liver metastatic foci compared to the control group (Fig. 1b, d).
Given some previous studies have shown the involvement of PLEK2 in actin remodeling, the morphological change was detected following PLEK2 knockdown or overexpression. Consistent with previous studies, PLEK2 knockdown cells displayed small and round shapes whereas PLEK2 overexpression cells exhibited spindle-like shapes compared to control cells (Fig. 1e). The change in the cell morphology might facilitate their motility. As the EMT process plays an indispensable role in tumor metastasis, whether the function of PLEK2 in cell spreading promoted the EMT process was investigated. As shown in Fig. 1f, PLEK2 knockdown suppressed Fibronectin and N-cadherin, whereas enhanced E-cadherin expression. On the contrary, PLEK2 overexpression enhanced Fibronectin and N-cadherin, whereas suppressed E-cadherin expression.
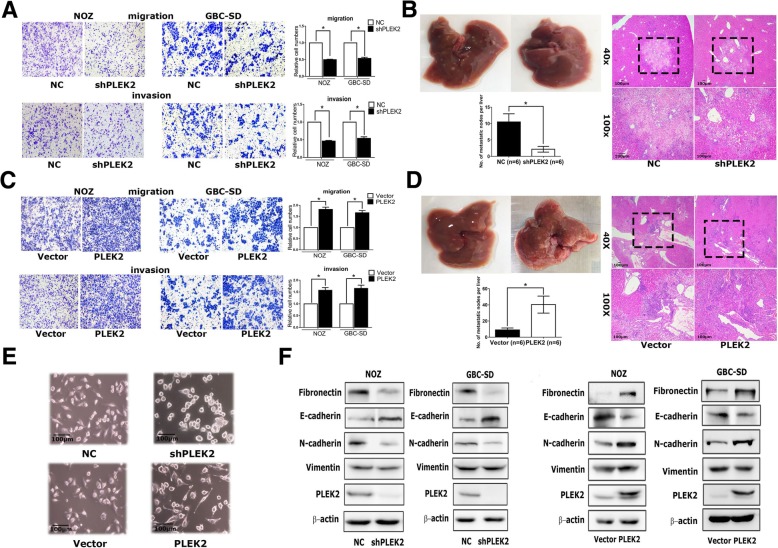 Fig.
1 Effects of PLEK2 on the migration, invasion, and metastasis of GBC cells. (Shen H, et al, 2019)
Fig.
1 Effects of PLEK2 on the migration, invasion, and metastasis of GBC cells. (Shen H, et al, 2019)
Silencing ARAF Inhibits GBC Cell Proliferation, Migration and Invasion
ARAF is a member of the RAF kinase family that is necessary for mitogen-activated protein kinase (MAPK) activation in various malignancies, including lung, colorectal, pancreatic, and breast cancers. As the most common biliary tract tumor, gallbladder cancer (GBC) seriously harms human health while the function of ARAF in GBC remains elusive.
ARAF knockdown was employed to study its function in GBC cells. ARAF expression was examined in GBC-SD and SGC-996 cells transfected with ARAF siRNA or siRNA control. After siRNA transfection, ARAF mRNA and protein levels were both significantly lower than controls (Fig. 2a and 2b). It was demonstrated that the ARAF siRNA successfully silenced endogenous ARAF in GBC cells. Inhibiting the rapid growth of cancer cells is an important way to treat cancers. As shown in Fig. 2c, the CCK-8 assays showed that ARAF siRNA inhibited the proliferation of different GBC cell lines, including GBC-SD cells and SGC-996 cells. Interestingly, PCNA, a reliable indicator of cell proliferation, was also higher in the control siRNA group compared with ARAF knockdown (Fig. 2d). Furthermore, colony formation was significantly reduced in the ARAF siRNA group, compared with controls (Fig. 2e and 2f).
The role of ARAF on the migration and invasion of GBC cells was further explored by wound healing and transwell assays. As shown in Fig. 3a and 3b, ARAF knockdown significantly attenuated cell migration compared with controls. The invasiveness of SGC-996 cells is too poor to use for transwell assays, GBC-SD cells were employed to investigate the role of ARAF on cell invasion. The invasion of GBC-SD cells was remarkably suppressed after ARAF was knocked down (Fig. 3c). These results indicate that silencing ARAF inhibits the migration and invasion of GBC cells.
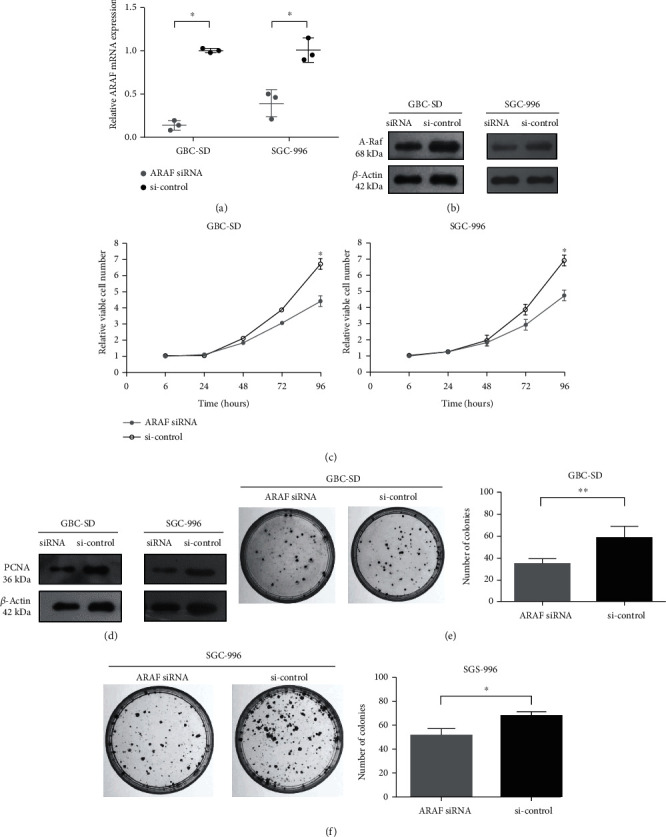 Fig.
2 Downregulated expression of ARAF inhibits GBC cell proliferation. (Lin W, et al., 2020)
Fig.
2 Downregulated expression of ARAF inhibits GBC cell proliferation. (Lin W, et al., 2020)
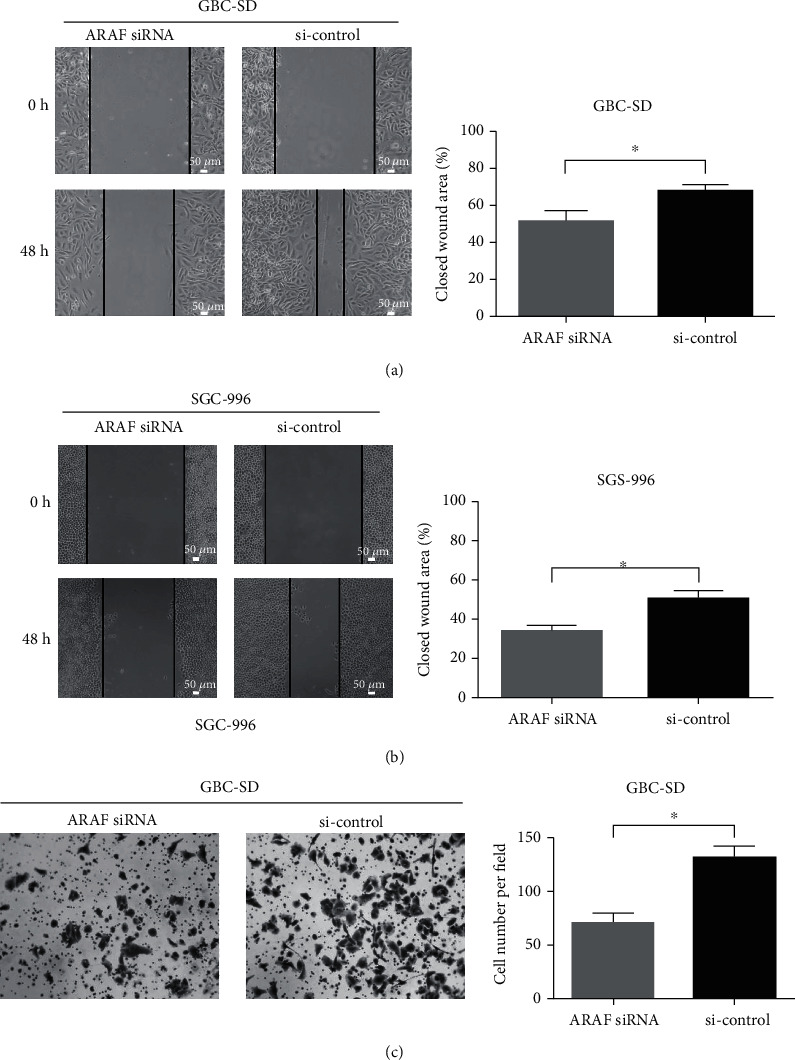 Fig.
3 ARAF promotes migration and invasion of GBC cells. (Lin W, et al., 2020)
Fig.
3 ARAF promotes migration and invasion of GBC cells. (Lin W, et al., 2020)
Gallbladder tumors can be classified into various types, including adenocarcinomas (the most common), squamous cell carcinomas, neuroendocrine tumors, and adenosquamous carcinomas.
Research on gallbladder tumor cells can lead to the discovery of novel therapeutic targets, biomarkers for early detection and prognosis, personalized treatment approaches, and a deeper understanding of the molecular mechanisms driving tumor development and progression.
Gallbladder tumor cells often exhibit altered metabolic profiles, including increased glycolysis, glutamine metabolism, and lipid synthesis. These metabolic adaptations allow gallbladder tumor cells to support their rapid proliferation and survive in the tumor microenvironment.
Filters Clear all filters
Species
- African clawed frog (1)
- American mink (1)
- Asian tiger mosquito (1)
- Atlantic salmon (1)
- Bluegill (2)
- Bluestriped grunt (1)
- Bovine (7)
- Brazilian free-tailed bat (1)
- Brown bullhead (2)
- Cabbage looper (1)
- Cabbage moth (6)
- Cat (4)
- Central mudminnow (1)
- Chicken (3)
- Chinese hamster (5)
- Chinook salmon (2)
- Chum salmon (1)
- Coho salmon (1)
- Common carp (2)
- Cotton-top tamarin (1)
- Dog (2)
- Fall armyworm (3)
- Fathead minnow (2)
- Fruit fly (1)
- Gilthead sea bream (2)
- Golden hamster (7)
- Goldfish (6)
- Gray dwarf hamster (1)
- Green monkey (2)
- Gypsy moth (1)
- Horse (1)
- Human (998)
- Japanese eel (1)
- Japanese rice fish (7)
- Koi carp (1)
- Mouse (315)
- Mouse x Gray dwarf hamster (1)
- Mouse x Rat (20)
- Northern pike (1)
- Pig (3)
- Rabbit (2)
- Rainbow trout (3)
- Rat (115)
- Rhesus macaque (1)
- Salt marsh moth (1)
- Sheep (2)
- Snakehead murrel (2)
- Sockeye salmon (1)
- Vervet monkey (2)
- Zebrafish (2)
Source
- Abdomen (1)
- Abdomen Metastasis (2)
- Adipose (2)
- Adrenal Gland (8)
- Adrenal Gland Metastasis (2)
- Aorta (4)
- Artery (1)
- Ascites (28)
- Ascites Metastasis (37)
- Bile Duct (3)
- Bladder (25)
- Bladder Metastasis (1)
- Blastocyst (1)
- Blastula (1)
- Blood (127)
- Bone (27)
- Bone Marrow (57)
- Bone Marrow Metastasis (18)
- Bone Metastasis (6)
- Brain (55)
- Brain Metastasis (8)
- Breast (30)
- Bronchus (1)
- Caudal Peduncle (1)
- Caudal Trunk (2)
- Cecum (3)
- Cerebrospinal Fluid (1)
- Cerebrospinal Fluid Metastasis (1)
- Cervix (32)
- Colon (90)
- Connective Tissue (7)
- Cornea (3)
- Cutaneous Metastasis (1)
- Dermis (2)
- Duodenum (1)
- Embryo (29)
- Endometrium (17)
- Esophagus (44)
- Eye (12)
- Eye Socket (5)
- Fetus (3)
- Fin (9)
- Foreskin (4)
- Gallbladder (1)
- Gingiva (2)
- Globe (2)
- Glomerulus (2)
- Groin (1)
- Head Kidney (2)
- Heart (4)
- Hemolymph (1)
- Hypodermis Metastasis (5)
- Ileum (1)
- Intestine (94)
- Jejunum (1)
- kidney (1)
- Kidney (27)
- Liver (35)
- Liver Metastasis (17)
- Lung (58)
- Lung Metastasis (8)
- Lymph Node (7)
- Lymph Node Metastasis (59)
- Muscle (7)
- Muscle Metastasis (2)
- Nose (2)
- Omentum Metastasis (2)
- Oral Cavity (10)
- Ovary (21)
- Ovary Metastasis (2)
- Pancreas (19)
- Pelvic Wall Metastasis (1)
- Pelvis (1)
- Perianal Space Metastasis (1)
- Pericardial Effusion (1)
- Pericardial Effusion Metastasis (2)
- Perineus (1)
- Peripheral Blood (126)
- Peripheral Nervous System (21)
- Peritoneal Effusion (2)
- Peritoneum (1)
- Peritoneum Metastasis (1)
- Pharynx (3)
- Pituitary Gland (7)
- Pleural Effusion (54)
- Pleural Effusion Metastasis (46)
- Prostate (7)
- Rectum (15)
- Renal Pelvis (1)
- Retroperitoneal Space (2)
- Salivary Gland (2)
- Skeletal Muscle (5)
- Skin (32)
- Skin Metastasis (3)
- Small Intestine (4)
- Small Intestine Metastasis (1)
- Smooth Muscle (2)
- Soft Tissue (1)
- Soft Tissue Metastasis (1)
- Spinal Cord (2)
- Stomach (4)
- Testis (15)
- Thoracic Cavity Metastasis (6)
- Thymus (5)
- Thyroid Gland (16)
- Thyroid Gland Metastasis (1)
- Tongue (5)
- Trachea (1)
- Umbilical Cord (1)
- Umbilical Cord Blood (1)
- Urachus (1)
- Ureter (1)
- Uterus (54)
- Uvea (2)
- Vagina (2)
- Vulva (1)
Disease
- Acute Biphenotypic Leukemia (1)
- Acute Erythroid Leukemia (4)
- Acute Megakaryoblastic Leukemia (4)
- Acute Monocytic Leukemia (9)
- Acute Myeloid Leukemia (25)
- Acute Promyelocytic Leukemia (2)
- Adrenal Gland Neuroblastoma (11)
- Adult B Acute Lymphoblastic leukemia (1)
- Adult B Acute Lymphoblastic Leukemia (6)
- Adult T Acute Lymphoblastic Leukemia (6)
- Adult T Lymphoblastic Lymphoma (2)
- Adult T-Cell Leukemia/Lymphoma (1)
- Alveolar Rhabdomyosarcoma (4)
- Alveolar Ridge Squamous Cell Carcinoma (1)
- Amelanotic Melanoma (3)
- Ampulla of Vater Adenocarcinoma (1)
- Ampulla of Vater Adenosquamous Carcinoma (3)
- Anaplastic Astrocytoma (3)
- Anaplastic Large Cell Lymphoma (7)
- Askin Tumor (1)
- Astrocytoma (5)
- B Acute Lymphoblastic Leukemia (2)
- B-Cell Non-Hodgkin Lymphoma (5)
- Bare Lymphocyte Syndrome Type 2 (1)
- Barrett Adenocarcinoma (2)
- Benign Prostatic Hyperplasia (1)
- Bladder Carcinoma (14)
- Bladder Squamous Cell Carcinoma (1)
- Bovine Leukemia (2)
- Breast Adenocarcinoma (4)
- Breast Carcinoma (9)
- Breast Ductal Carcinoma (2)
- Burkitt Lymphoma (17)
- Canavan Disease (1)
- Canine Histiocytic Sarcoma (1)
- Cecum Adenocarcinoma (3)
- Central Nervous System Lymphoma (2)
- Cervical Adenocarcinoma (2)
- Cervical Adenosquamous Carcinoma (2)
- Cervical Small Cell Carcinoma (1)
- Cervical Squamous Cell Carcinoma (2)
- Chicken Bursal Lymphoma (2)
- Childhood B Acute Lymphoblastic Leukemia (13)
- Childhood T Acute Lymphoblastic Leukemia (16)
- Childhood T Lymphoblastic Lymphoma (1)
- Cholangiocarcinoma (2)
- Chronic Eosinophilic Leukemia (1)
- Chronic Lymphocytic Leukemia (2)
- Chronic Myeloid Leukemia (23)
- Clear Cell Renal Cell Carcinoma (2)
- Colon Adenocarcinoma (55)
- Colon Carcinoma (34)
- Colorectal Adenocarcinoma (1)
- Colorectal Carcinoma (1)
- Congenital Pure Red Cell Aplasia (1)
- Cutaneous Melanoma (10)
- Dedifferentiated Chondrosarcoma (1)
- Desmoplastic Melanoma (1)
- Diffuse Large B-Cell Lymphoma (28)
- Down Syndrome (2)
- EBV-Related Burkitt Lymphoma (12)
- Embryonal Carcinoma (3)
- Embryonal Rhabdomyosarcoma (3)
- Endometrial Adenocarcinoma (13)
- Endometrial Adenosquamous Carcinoma (2)
- Endometrial Carcinoma (2)
- Endometrioid Stromal Sarcoma (1)
- Epithelioid Hemangioendothelioma (1)
- Epithelioid Sarcoma (3)
- Esophageal Adenocarcinoma (6)
- Esophageal Squamous Cell Carcinoma (41)
- Essential Thrombocythemia (1)
- Ewing Sarcoma (2)
- Extraskeletal Myxoid Chondrosarcoma (1)
- Fanconi Anemia (1)
- Fibrosarcoma (1)
- Follicular Lymphoma (2)
- Gallbladder Carcinoma (2)
- Gallbladder Undifferentiated Carcinoma (2)
- Gastric Adenocarcinoma (6)
- Gastric Adenosquamous Carcinoma (1)
- Gastric Carcinoma (5)
- Gastric Choriocarcinoma (1)
- Gastric Fundus Carcinoma (1)
- Gastric Signet Ring Cell Adenocarcinoma (1)
- Gastric Small Cell Carcinoma (2)
- Gastric Tubular Adenocarcinoma (5)
- Gastroesophageal Junction Adenocarcinoma (1)
- Gestational Choriocarcinoma (1)
- Gingival Squamous Cell Carcinoma (2)
- Glioblastoma (18)
- Gliosarcoma (1)
- Goldfish Erythrophoroma (4)
- Hairy Cell Leukemia (1)
- Hamster Kidney Tumor (1)
- Hamster Pancreatic Ductal Adenocarcinoma (1)
- Hamster Uterine Leiomyosarcoma (1)
- Hepatoblastoma (2)
- Hepatocellular Carcinoma (6)
- Hepatosplenic T-Cell Lymphoma (2)
- Hereditary Thyroid Gland Medullary Carcinoma (1)
- High Grade B-Cell Lymphoma (1)
- High Grade Ovarian Serous Adenocarcinoma (8)
- Hodgkin Lymphoma (9)
- Hypopharyngeal Squamous Cell Carcinoma (2)
- Infectious Mononucleosis (1)
- Intrahepatic Cholangiocarcinoma (6)
- Invasive Breast Carcinoma of No Special Type (12)
- Invasive Breast Lobular Carcinoma (1)
- Kidney Neoplasm (1)
- Kidney Rhabdoid Tumor (1)
- Krukenberg Tumor (1)
- Liposarcoma (1)
- Lung Adenocarcinoma (17)
- Lung Giant Cell Carcinoma (8)
- Lung Large Cell Carcinoma (9)
- Lung Mucoepidermoid Carcinoma (1)
- Lung Non-Small Cell Carcinoma (2)
- Lung Small Cell Carcinoma (25)
- Lung Squamous Cell Carcinoma (9)
- Lymphoblastic Lymphoma (1)
- Malignant Peripheral Nerve Sheath Tumor (1)
- Mantle Cell Lymphoma (5)
- Mature Gastric Teratoma (1)
- Maxillary Sinus Squamous Cell Carcinoma (1)
- Medaka Hepatoma (2)
- Medulloblastoma (3)
- Melanoma (24)
- Meningioma (2)
- Minimally Invasive Lung Adenocarcinoma (1)
- Monophasic Synovial Sarcoma (1)
- Mouse Bladder Transitional Cell Carcinoma (1)
- Mouse Chondrosarcoma (1)
- Mouse Colon Adenocarcinoma (3)
- Mouse Ependymoma (2)
- Mouse Erythroid Leukemia (13)
- Mouse Fibrosarcoma (5)
- Mouse Glioblastoma (1)
- Mouse Hemangioendothelioma (1)
- Mouse Hepatocellular Carcinoma (1)
- Mouse Insulinoma (3)
- Mouse Intestinal Tract Neuroendocrine Adenoma (1)
- Mouse Islet Cell Adenoma (1)
- Mouse Kidney Carcinoma (1)
- Mouse Leukemia (10)
- Mouse Leydig Cell Tumor (1)
- Mouse Lymphoma (8)
- Mouse Mammary Gland Malignant Neoplasm (23)
- Mouse Melanoma (9)
- Mouse Multiple Myeloma (5)
- Mouse Myeloid Leukemia (3)
- Mouse Neoplasm (1)
- Mouse Neuroblastoma (21)
- Mouse Oral Cavity Squamous Cell Carcinoma (1)
- Mouse Osteosarcoma (3)
- Mouse Pituitary Gland Neoplasm (1)
- Mouse Plasmacytoma (1)
- Mouse Precursor T Cell Lymphoblastic Lymphoma/Leukemia (2)
- Mouse Pulmonary Adenoma (1)
- Mouse Pulmonary Malignant Tumor (3)
- Mouse Pulmonary Squamous Cell Carcinoma (1)
- Mouse Rectum Carcinoma (2)
- Mouse Reticulum Cell Sarcoma (2)
- Mouse Sarcoma (1)
- Mouse Teratocarcinoma (8)
- Mouse Thymic Lymphoma (3)
- Mycosis Fungoides (1)
- Myelodysplastic Syndrome (1)
- Myxofibrosarcoma (1)
- Natural Killer Cell Lymphoblastic Leukemia/Lymphoma (2)
- Neuroblastoma (26)
- Oral Cavity Squamous Cell Carcinoma (15)
- Osteoid Osteoma (1)
- Osteosarcoma (15)
- Ovarian Carcinoma (1)
- Ovarian Clear Cell Adenocarcinoma (1)
- Ovarian Endometrioid Adenocarcinoma (4)
- Ovarian Granulosa Cell Tumor (1)
- Ovarian Mucinous Adenocarcinoma (2)
- Ovarian Serous Adenocarcinoma (2)
- Ovarian Serous Cystadenocarcinoma (2)
- Ovarian Small Cell Carcinoma (1)
- Pancreatic Adenocarcinoma (13)
- Pancreatic Carcinoma (5)
- Pancreatic Ductal Adenocarcinoma (12)
- Papillomavirus-Independent Cervical Squamous Cell Carcinoma (1)
- Papillomavirus-Related Cervical Adenocarcinoma (7)
- Papillomavirus-Related Cervical Squamous Cell Carcinoma (4)
- Papillomavirus-Related Endocervical Adenocarcinoma (16)
- Paroxysmal Nocturnal Hemoglobinuria (3)
- Pharyngeal Squamous Cell Carcinoma (1)
- Plasma Cell Myeloma (15)
- Pleural Epithelioid Mesothelioma (5)
- Pleural Sarcomatoid Mesothelioma (2)
- Poorly Differentiated Thyroid Gland Carcinoma (1)
- Primary Cutaneous T-Cell Non-Hodgkin Lymphoma (1)
- Primary Effusion Lymphoma (7)
- Primitive Neuroectodermal Tumor (1)
- Prostate carcinoma (1)
- Prostate Carcinoma (9)
- Rat C-Cell Carcinoma (1)
- Rat Cholangiocarcinoma (1)
- Rat Colon Adenocarcinoma (5)
- Rat Digestive System Neoplasm (1)
- Rat Fibrosarcoma (1)
- Rat Hepatocellular Carcinoma (20)
- Rat Histiocytic Sarcoma (1)
- Rat Insulinoma (2)
- Rat Leukemia (1)
- Rat Leydig Cell Adenoma (1)
- Rat Lung Carcinoma (1)
- Rat Malignant Glioma (4)
- Rat Malignant Meningioma (1)
- Rat Malignant Oligodendroglioma (2)
- Rat Malignant Thymoma (3)
- Rat Mammary Gland Adenocarcinoma (10)
- Rat Neuroblastoma (3)
- Rat Osteosarcoma (2)
- Rat Pituitary Gland Neoplasm (6)
- Rat Prostate Adenocarcinoma (3)
- Rat Rhabdomyosarcoma (1)
- Rat Sarcoma (2)
- Rat Squamous Cell Carcinoma (1)
- Rat Urinary Bladder Transitional Cell Carcinoma (2)
- Rat Urinary System Neoplasm (6)
- Rectal Adenocarcinoma (13)
- Rectosigmoid Adenocarcinoma (1)
- Recurrent Bladder Carcinoma (1)
- Renal Cell Carcinoma (7)
- Renal Pelvis Urothelial Carcinoma (1)
- Retinoblastoma (11)
- Sacral Chordoma (1)
- Sacrococcygeal Teratoma (1)
- Salivary Gland Squamous Cell Carcinoma (1)
- Sezary Syndrome (1)
- Shwachman-Diamond Syndrome (1)
- Skin Squamous Cell Carcinoma (2)
- Splenic Marginal Zone Lymphoma (1)
- Testicular Embryonal Carcinoma (8)
- Testicular Teratoma (2)
- Testicular Yolk Sac Tumor (1)
- Thyroid Gland Anaplastic Carcinoma (10)
- Thyroid Gland Follicular Carcinoma (4)
- Thyroid Gland Papillary Carcinoma (3)
- Thyroid Gland Sarcoma (1)
- Thyroid Gland Squamous Cell Carcinoma (2)
- Tongue Adenosquamous Carcinoma (1)
- Tongue Squamous Cell Carcinoma (6)
- Type I Endometrial Adenocarcinoma (1)
- Ureter Urothelial Carcinoma (1)
- Uterine Carcinosarcoma (2)
- Uterine Corpus Leiomyosarcoma (1)
- Uterine Corpus Sarcoma (2)
- Uveal Melanoma (2)
- Vaginal Melanoma (2)
- Vulvar Melanoma (1)
- Vulvar Squamous Cell Carcinoma (1)
Description: Japanese gallbladder carcinoma established from metastated ascite.
Description: Japanese gallbladder tumor passed through a nude mouse.
Description: Japanese gallbladder carcinoma from the same patient of TGBC1TKB.
Description: Japanese gallbadder carcinoma metastated to lymph node from the same patient of TGBC2TKB.
Description: Human poorly differentiated cholangiocarcinoma cell line established from biliary tract.
Description: Human mixed (papillary and non-papillary) cholangiocarcinoma cell line established from biliary ...
Description: Human cell line derived from cholangiocarcinoma.






