Exosome Size Measurement
In recent years, exosomes have garnered substantial attention in the field of life science research due to their pivotal role in intercellular communication and potential clinical applications. Exosomes, small extracellular vesicles of endocytic origin, are released by various cell types and carry a cargo of proteins, nucleic acids, and lipids. These nano-sized vesicles facilitate cellular communication and contribute to diverse physiological and pathological processes. Understanding the size of exosomes is crucial, as it directly impacts their functions and interactions with target cells.
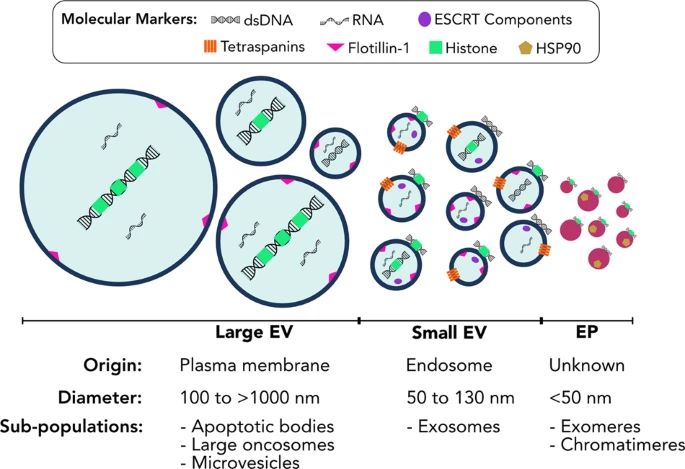 Fig. 1 Extracellular vesicles and particles comprise heterogeneous populations. (Malkin EZ, Bratman SV., 2020)
Fig. 1 Extracellular vesicles and particles comprise heterogeneous populations. (Malkin EZ, Bratman SV., 2020)
Exosome Size Measurement Techniques
Various techniques are employed for exosome size measurement, including electron microscopy, nanoparticle tracking analysis (NTA), dynamic light scattering (DLS), and resistive pulse sensing (RPS). These techniques offer distinct advantages in visualizing, quantifying, and characterizing exosome size distribution, providing valuable insights into their heterogeneity and biological relevance.
- Electron microscopy (EM). Electron microscopy, including transmission electron microscopy (TEM) and scanning electron microscopy (SEM), allows for direct visualization of exosomes at nanoscale resolution. This technique provides detailed morphological information and precise size determination of individual exosomes. EM is particularly valuable for observing the ultrastructure and size distribution of exosomes.
- Nanoparticle tracking analysis (NTA). NTA utilizes laser light scattering and Brownian motion analysis to track and size individual exosomes suspended in a liquid medium in real time. NTA provides particle-by-particle sizing and quantification, allowing for the determination of size distribution and concentration of exosomes within a sample. It is a widely used technique for rapid and accurate exosome size analysis.
- Dynamic light scattering (DLS). DLS measures the fluctuations in light scattering caused by the Brownian motion of exosomes in solution. This technique provides hydrodynamic size distributions of particles in the submicron range, allowing for the characterization of the size distribution and polydispersity of exosomes. DLS is valuable for evaluating the stability and uniformity of exosome preparations.
- Resistive pulse sensing (RPS) or tunable resistive pulse sensing (TRPS). RPS/TRPS involves the use of a nanopore and measures change in electrical resistance as exosomes pass through the pore one at a time. This method allows for the determination of the size and concentration of individual exosomes with high resolution. It is particularly useful for analyzing the size heterogeneity and concentration of exosomes within a sample.
- Tunable nanopore sensing (also known as resistive-pulse sensing). This technique measures the changes in ionic current as exosomes flow through a nanopore. By analyzing these changes, the size and concentration of exosomes can be determined. This technique is particularly beneficial for single-particle analysis and characterization of exosome size distribution.
Factors Affecting Exosome Size
The size of exosomes is influenced by a myriad of factors, including their cellular origin, biogenesis pathways, and the surrounding microenvironment. For instance, exosomes derived from different cell types exhibit variability in size due to the unique intracellular processes governing their biogenesis. Environmental factors, such as pH, temperature, and oxidative stress, can also impact exosome size. Understanding these determinants is crucial for interpreting the functional implications of exosome size heterogeneity in physiological and pathological contexts.
- Cellular origin. Exosomes are released by diverse cell types, and their biogenesis is intricately linked to the cellular machinery involved in endocytosis and multivesicular body (MVB) formation. Different cell types may produce exosomes with varying sizes due to distinct biogenesis processes and intracellular vesicle sorting mechanisms. As a result, exosomes derived from different cell types may exhibit heterogeneity in size and cargo composition.
- Biogenesis pathways. The biogenesis of exosomes involves the inward budding of the endosomal membrane, leading to the formation of intraluminal vesicles within MVBs. The size of exosomes can be influenced by the regulatory mechanisms governing the biogenesis and sorting of cargo into intraluminal vesicles. Variations in the biogenesis pathways, such as endosomal sorting complexes required for transport (ESCRT)-dependent or ESCRT-independent mechanisms, can impact the size and cargo content of exosomes.
- Environmental conditions. The microenvironment in which cells reside can impact exosome biogenesis and secretion. Factors such as pH, temperature, oxidative stress, and extracellular signaling molecules can influence the size of exosomes. For example, exposure to stress conditions may alter the biophysical properties of exosomes and lead to changes in size distribution.
- Cellular stress and activation. Cellular stress, such as hypoxia, inflammation, or cellular activation, can modulate the size and cargo of exosomes. Under stress conditions, cells may release a distinct population of exosomes with altered size profiles, reflecting the adaptive responses of the parent cells to the microenvironmental cues.
- Physiological states. Exosome size may also vary based on the physiological state of the parent cells. For instance, during processes such as cell proliferation, differentiation, or apoptosis, the release of exosomes with specific size characteristics may be modulated in response to the cellular state.
- Extracellular vesicle heterogeneity. In addition to exosomes, other types of extracellular vesicles, such as micro-vesicles and apoptotic bodies, contribute to the overall vesicle population released by cells. The heterogeneity of extracellular vesicles may impact the size distribution of the isolated vesicle population, necessitating careful characterization to distinguish exosomes based on their size and biogenesis pathways.
Applications of Exosome Transfection in Biomedical Research
Accurate measurement of exosome size holds promising implications for clinical diagnostics, biomarker discovery, and therapeutic interventions. For example, in cancer research, the size distribution of tumor-derived exosomes has been linked to disease progression and metastatic potential. Furthermore, the development of exosome-based biomarkers for various diseases, including neurodegenerative disorders, cardiovascular diseases, and infectious conditions, relies on precise size characterization to elucidate their diagnostic and prognostic value.
Creative Bioarray Relevant Recommendations
| Product/Service Types | Description |
| Exosome Standards | Creative Bioarray provides the best quality lyophilized exosome standards obtained from several biological samples, including hundreds of different cell lines, plasma, serum, saliva, and urine as well as other bio-fluids. |
| Exosome Isolation and Purification | Creative Bioarray provides reliable techniques for exosome isolation from different sample matrices. We also provide exosome isolation using specified techniques if required by customers. |
| Exosome Identification | Creative Bioarray's exosome identification service makes it easy to detect and characterize exosomes with our experience working on exosomes. Our expertise characterizes isolated exosomes in many different ways including particle size, quantity, specific antibodies, and more. |
| Exosome Quantification | Creative Bioarray offers a range of options to meet most exosome quantitation demands. We provide reliable, and optimized tools at the most competitive price for quantitative analysis of exosomes in diverse biological samples such as plasma, urine, etc. |
| Exosome Analysis | Creative Bioarray provides diverse exosomal species analysis to help you understand your exosome compositions. We provide RNA-seq, proteomics, lipidomics, and metabolomics analyses. |
Reference
- Malkin EZ, Bratman SV. (2020). "Bioactive DNA from extracellular vesicles and particles." Cell Death Dis. 11 (7), 584.