CAR-T/CAR-NK Target Assessment Service (ISH)
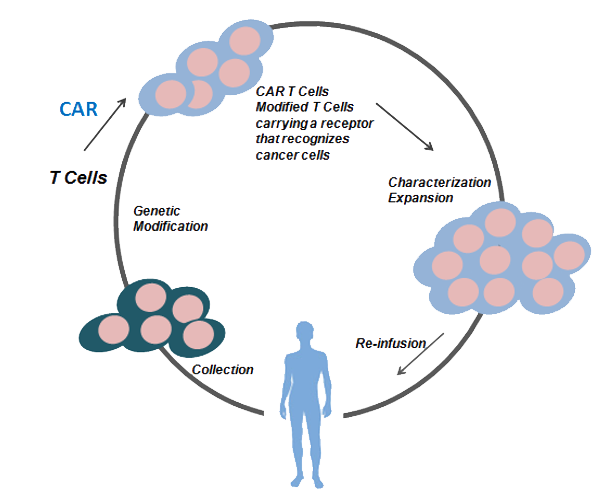
Chimeric antigen receptor (CAR) T cell therapy is highly effective in treating hematologic malignancies, and major efforts are being made to achieve similar efficacy in solid tumors. Two landmark events occurred for CAR-T cell therapy in 2017: two anti-CD19 CAR-T cells were approved for the therapy of acute lymphoblastic leukemia (ALL) and relapsed or refractory large B-cell lymphoma, respectively. From then, increasing number of researchers have been dedicated to CAR-T cell therapy research around the world. In CAR-T cell therapies the collection of patient's T cells is followed by a genetic modification of these immune cells to obtain the expression of chimeric antigen receptors specific for the tumor cell of interest. After the characterization and expansion ex vivo, they are re-infused to the patient to fight against the patient's tumor cells.
Toxicity Assessment of CAR-T Cells
Since CAR T therapeutics have entered the clinic, several toxic side effects have been identified. "On-target/off-tumor" toxicity was an expected side effect of CAR T therapy from the outset. "on-target/off-tumor" toxicity occurs when the CAR T cells attack non-tumor cells that express the intended target antigen. However, the current generation of CAR-T cells cannot distinguish between neolastic and normal cells that may also be expressing the same antigen, thus potentially resulting in "on-target/off- tumor" toxicity. In Situ Hybridization (ISH) is a great method that can be utilized to identify novel CAR-T cell targets and subsequently qualify monoclonal antibodies directed against those targets for immunohistochemistry. ISH can be used to predict CAR-T cell target organ toxicity in preclinical models.
Disrtribution Assessment of CAR-T Cells
The distribution of CAR-T cells in the tumor and in the body is important for assessing the antitumor effect and safety of the injected CAR-T in vivo, while observing the antitumor effect. Because of the heterogeneity of the tumor, the in situ localization and distribution of CAR-T cells in tumor cells and tumor microenvironment can reflect the mechanism and application prospect of the treatment method. Creative Bioarray can help you in situ detect the distribution of CAR-T cells in tumor cells or tissues. ISH can be used to evaluate the CAR expression.
As CAR-T Cells carry their own unique nucleic acid sequences, RNA specific probes can be designed for these regions to effectively detect the distribution of CAR-T Cells. So RNA ISH is a unique and effective tool for in situ detecting CAR T cells. Notably, we have generated CAR-T ISH probes not only for blood cancer antigen CD19, but also for solid tumors against different antigens, such as Her2, Her3, EGFR, FGFR1, VEGFR, etc.
As a leading technology provider, Creative Bioarray's elite scientists have broad experience in CAR-T/CAR-NK Target Assessment (ISH), which can supply the best service for you.
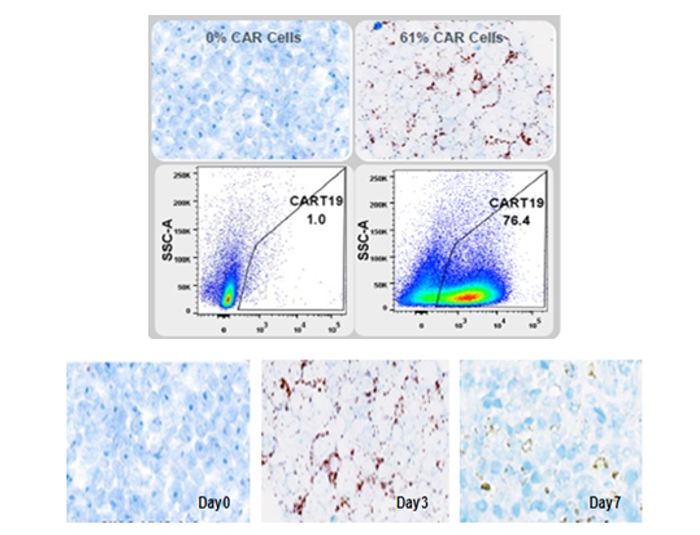 Figure 2. The in situ localization and distribution of CAR T Cells detection by RNA ISH.
Figure 2. The in situ localization and distribution of CAR T Cells detection by RNA ISH.
Applications
- In situ detect the distribution of CAR-T cells in tumor cells or tissues
- Assess the antitumor effect and safety of the CAR-T cells
- Streamline your CAR-T target identification and selection workflows
- Evaluate the target expression in normal tissues can be used to predict CAR-T cell induced toxicity
- Assess target expression specificity and to track CAR-T cell distribution and activation in xenograft and host tissues
Features
- Accurate - In Situ Detection Service-Custom design your probe
- Value- We focus on the quality of our service and all supported by competitive pricing
- Efficiency - We are able to provide the fastest turnaround time of any supplier in the industry
Creative Bioarray offers custom CAR-T/CAR-NK Target Assessment service (ISH) for you as follows:
- Probe design
- Probe synthesis
- ISH
- Imaging
- Data analysis
Quotations and ordering
Our customer service representatives are available 24hr a day! We thank you for choosing Creative Bioarray at your preferred CAR-T/CAR-NK Target Assessment service (ISH).
References
Haile S T,; et al. Attenuated oncolytic virus HSV1716 increases in vivo expansion of GD2-targeting CAR T cells in murine solid tumor models[J]. AACR 107th Annual Meeting 2016; April 16-20, 2016.
Zhu Y,; et al. Anti-CD19 chimeric antigen receptor T-cell therapy for adult Philadelphia chromosome-positive acute lymphoblastic leukemia: two case reports[J]. Medicine, 2016, 95(51).
Wing A,; et al. Improving CART-cell therapy of solid tumors with oncolytic virus–driven production of a bispecific T-cell engager[J]. Cancer immunology research, 2018, 6(5): 605-616.
Explore Other Options