Guides for Live Cell Imaging Dyes
Live cell imaging has revolutionized the field of cell biology, allowing researchers to study cellular processes and responses in real-time, within living cells. This technique has greatly benefited from the development of fluorescent dyes, which enable visualization of specific cellular structures and processes.
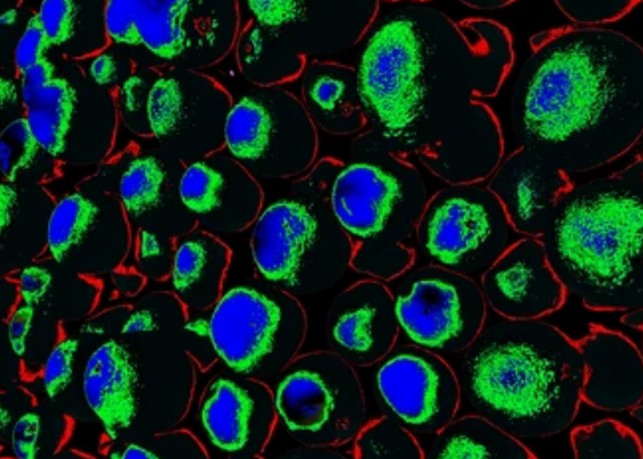
Principles of Live Cell Imaging and Fluorescent Labeling
Live cell imaging is based on visualizing cells or cellular processes within a live, unperturbed environment. Fluorescent labeling is a fundamental technique used to achieve this visualization. By attaching a fluorescent dye to a specific cellular component or molecule, researchers can observe its behavior and interactions with other cellular elements in real time.
Fluorescent labeling relies on the properties of fluorescent dyes, which absorb light at a specific wavelength and emit light at a longer wavelength, producing a visible fluorescent signal. This allows for the selective visualization of specific cellular structures or molecules within the complex environment of a live cell.
Classification of Live Cell Imaging Dyes Based on Applications
- Fluorescent protein-based live cell imaging dyes. Fluorescent proteins, such as green fluorescent protein (GFP) and its derivatives, have become indispensable tools for live cell imaging. The use of fluorescent proteins offers the advantage of specific and stable labeling without the need for exogenous dyes.
- Small molecule-based live cell imaging dyes. Small molecule-based dyes are commonly used for live cell imaging due to their versatility and compatibility with various labeling strategies, which can be targeted to specific cellular compartments or biomolecules, enabling high-resolution imaging of dynamic processes within live cells.
- Live cell imaging dyes for organelle-specific labeling. Specialized live cell imaging dyes have been developed for specific organelle labeling, such as mitochondria, endoplasmic reticulum, and lysosomes. These dyes enable the visualization of organelle dynamics and interactions, providing valuable insights into cellular function and pathology.
Techniques for Live Cell Imaging with Fluorescent Dyes
- Widefield microscopy. Coupled with fluorescent dyes, it allows for the visualization of cellular structures and processes in live cells. This technique provides a broad field of view, making it suitable for imaging large areas of the cell. Time-lapse imaging with widefield microscopy is valuable for observing dynamic processes over extended periods and capturing temporal changes in cellular behavior.
- Confocal microscopy. It offers superior optical sectioning capabilities, enabling the acquisition of sharp, high-contrast images of fluorescently labeled cellular structures at different focal planes within the specimen. This technique is particularly useful for imaging thick samples and obtaining three-dimensional reconstructions of cellular architecture.
- Super-resolution microscopy. Super-resolution microscopy techniques, such as structured illumination microscopy (SIM), stimulated emission depletion microscopy (STED), and single-molecule localization microscopy (SMLM), break the diffraction limit of light, enabling the visualization of cellular structures at nanoscale resolution. These techniques provide unprecedented detail and clarity, allowing researchers to unravel the intricacies of molecular interactions and spatial organization within live cells.
- FRET and FLIM imaging. Fluorescence resonance energy transfer (FRET) and fluorescence lifetime imaging microscopy (FLIM) are advanced imaging techniques that enable the study of molecular interactions and conformational changes within live cells. By employing fluorescent protein or dye pairs, FRET imaging can monitor real-time protein-protein interactions, while FLIM can measure the fluorescence lifetime of a fluorophore, providing quantitative information about its local environment and dynamics.
Precautions for Using Live Cell Imaging Dyes
- Dye toxicity. Careful assessment of the potential toxicity of the imaging dyes is essential. High concentrations or prolonged exposure to certain dyes can perturb cellular function and viability, leading to unintended experimental artifacts. Therefore, it is imperative to optimize the dye concentration and exposure time to minimize potential cytotoxic effects.
- Nonspecific binding. Some fluorescent dyes may exhibit nonspecific binding to cellular components, leading to background fluorescence and false-positive signals. In such cases, thorough validation of dye specificity through control experiments and colocalization studies is essential to distinguish true labeling from nonspecific interactions.
- Optimization of labeling protocol. Optimization of the labeling protocol, including incubation time, temperature, and washing steps, is critical to ensure efficient and specific labeling of the target structures or molecules within live cells. Rigorous experimental optimization can help minimize variability and enhance the reproducibility of live cell imaging data.
- Imaging conditions. Proper calibration of imaging parameters, such as laser power, detector sensitivity, and imaging depth, is paramount to obtain high-quality data while minimizing potential photo-induced damage to the cells. These imaging conditions should be carefully controlled and standardized across experiments to enable meaningful comparisons and reliable interpretation of results.
Creative Bioarray Relevant Recommendations
As a leading biological company with a rich history of innovation and expertise, Creative Bioarray specializes in providing a comprehensive array of live cell imaging dyes designed to support cutting-edge research in cell biology and biomedical sciences.
View our full range of live cell imaging dyes and find what you need!