Contamination of Cell Cultures & Treatment
Though contamination is a major factor to influence the cell growth and proliferation, there are series of methods to prevent or eliminate contamination. In normal conditions, the treatment of suspected infection may just discard a single potentially infected culture. But if the contamination problem continuously exists, it would be totally different – isolation from other laboratories, thoroughly clean, and sterility tests are absolutely essential. Good training of staff, attention to laboratory layout, appropriate use of quarantine for new cultures or cell lines, regular cleaning and maintenance, and quality control are important factors in preventing contamination in cell culture laboratories.
Sources and Forms of Contamination

Operator
The human operator is potentially the greatest hazard in the laboratory. Sloughing of skin scales from laboratory workers constantly seeds the environment with microorganisms and with the high levels of commensal microbes (i.e. normal inhabitants) in parts of the human mouth even just talking will generate potentially contaminating aerosols. Furthermore, an individual laboratory worker with an infection will represent a much higher risk source of contamination.

Environment
Materials and staff coming into the laboratory and points of access to the environment (drains, ventilation grills, and unsealed ceiling voids) will inevitably provide a constant supply of fresh contamination which will accumulate on laboratory surfaces and multiply in damp areas (e.g. fridges, sinks, waterbaths, and humidified incubators).

Regents and Equipment
Clearly procedural and physical control of materials coming into the laboratory, laboratory cleaning and aseptic technique will all be important activities that combined, will help to keep cell cultures free of contamination.
Other Cell Cultures
For primary cells, isolated directly from animal tissues, or their early passages are at a particularly high risk of contamination as the dissection process may promote contamination from the natural commensal flora in certain tissues and any subclinical infection.
Two main categories of cell culture contaminants
| Chemical Contaminants | Biological Contaminants |
| Impurities in media, Sera, and Water, Endotoxins, Plasticizers, and Detergents | Bacteria, Molds, Yeasts, Viruses, Mycoplasma, As well as cross contamination by other cell lines |
Invariably bacterial or fungal contamination will result in total loss of the affected culture. Recovery of cell lines from such overwhelming contaminants is undesirable as the contamination may survive in spite of antibiotic treatment only to reemerge later and in some cases with increased antibiotic resistance. Eradication of fungi is especially difficult as effective antifungal agents are often cytotoxic to the cell line and fungal spores are much more likely to persist in cultures and the environment only to cause intractable problems later.
Mycoplasma are very small Mollicutes (not bacteria), they typically grow adhering to the cell membrane which are a widespread cause of cell culture contamination, originally arising from animal tissues, reagents of animal origin and even human operators. Mycoplasma infection can spread rapidly in the laboratory as contamination may not be obvious to the operator (i.e. no medium turbidity), is resistant to commonly used antibiotics and can survive in the laboratory environment in aerosols and splashes of culture medium. Mycoplasma has been shown to cause numerous types of damaging and permanent changes in cell lines.

Persistent viral infections of cell lines may represent an infection risk to laboratory workers and this risk can also be significant for cell lines since they will clearly have some affect on the cell biology which could influence the validity of any data generated with that culture and could affect their performance and acceptability for manufacturing purposes.
Endotoxins are the lipopolysaccaride-containing by-products of gram-negative bacteria, which are another source of chemical contaminants in cell culture systems. They are commonly found in water, sera and some culture additives. They may affect the growth or performance of cultures and are a significant source of experimental variability. Furthermore, since the use of cell culture produced therapeutics, such as hybridomas and vaccines, are compromised by high endotoxin levels, efforts must be made to keep endotoxin levels in culture systems as low as possible.
Responses to Contamination Incidents
Remedial Management of Contamination Events
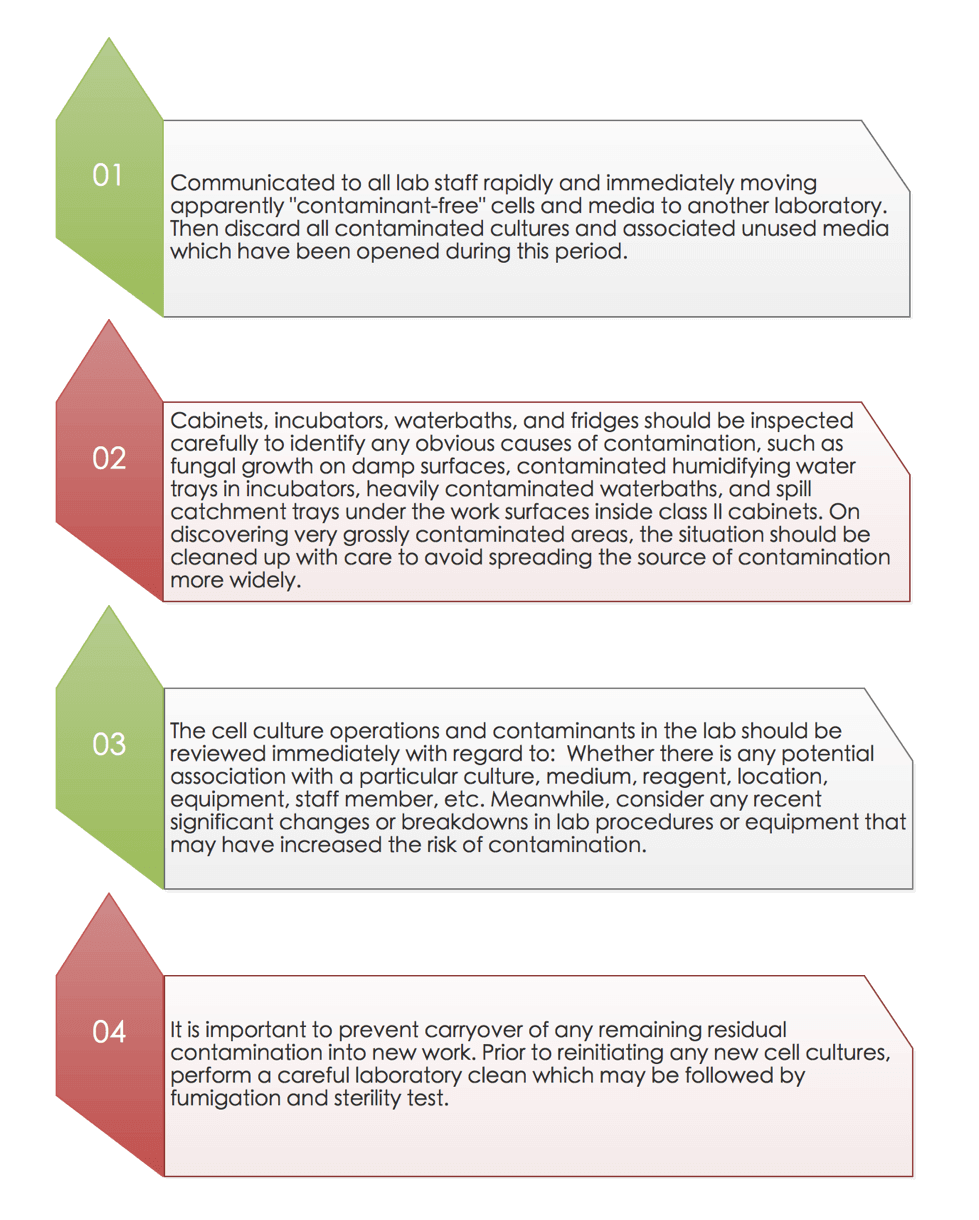
- When an overwhelming bacterial or fungal contamination or a dramatic and unexpected cytopathic affect or other suspicious morphological change arises in cell cultures, the immediate response should be to discard the affected cultures.
- The event should be notified to all other users of the lab who might have utilized the same cells, and reagents/media, so that they can be alert to any further contamination and if necessary be prepared to discard the reagents and cells they have been using.
- If only one or a very small number of flasks were affected from a batch of cultures and no other lab users have reported problems, it may be safe to assume that the contamination event arose due to chance introduction of organisms solely into the affected flasks and would be unlikely to recur.
Suspected Ongoing or General Source of Contamination
If the contamination appears to be more widespread in the laboratory or recurs on a number of occasions, a series of measures need to be taken to prevent further spread of the contamination as soon as possible.
Recovery and Monitoring
- The process of recovery from a major and/or widespread contamination incident will clearly demand careful monitoring to ensure that the original problem does not reappear. In the meantime, staff should be encouraged to report any further incidents or suspicious observations so that any recurrence can be captured and controlled rapidly.
- If a particular type of microorganism was implicated, it may be possible to implement specific testing that will flag up its reappearance and differentiate it more quickly from other general contamination. Such procedures could be discussed with a local diagnostic microbiology laboratory.
Avoidance and Prevention
Training - Staff training in aseptic technique will be the primary means to control contamination of cell cultures. Training in aseptic technique and identifying microbial contamination and appropriate disinfection and disposal of all routine waste will accordingly be vital and are an essential part of induction for new and inexperienced staff.
Laboratory Layout - The laboratory layout can be developed to reduce the risk of contamination by placing cell culture cabinets out of main thoroughfares, placing waste disposal collection away from clean work areas and sterile media storage and obviously segregating any work with (or storage of) microorganisms from preparation of clean cell cultures. Positioning of class II cabinets should be carefully planned to avoid interference by lab furniture/ equipment, walls, benching, doors, and other cabinets.
Cleaning and Maintenance - Laboratory cleaning is at the frontline of preventive maintenance where contamination is concerned. A formal routine cleaning regime for the general laboratory will help to control the levels of environmental contamination and thereby help to reduce the risks of day-to-day contamination.
Routine Quality Control - Establishment of a cell banking regime that will provide a stock of low passage cultures that have been subjected to repeated quality control tests is a fundamental aspect of good cell culture practice. Periodic testing of cell lines in use in the lab will give confidence that occult contamination, notably Mycoplasma, do not go unrecognized and where they do arise, are dealt with quickly so that the impact on any resulting work is minimised.
Summary
Even in the best laboratories contaminations do arise, the following procedure is recommended:
(1) Good training for cell culture procedure.
(2) Check for contamination by eye and with a microscope at each handling of a culture. Check for mycoplasma every month.
(3) If the contamination is new and is not widespread, discard the culture, the medium bottle used to feed it, and any other reagent (e.g., trypsin) that has been used in conjunction with the culture. Discard all of these into disinfectant, preferably in a fume hood and outside the tissue culture area.
(4) If the contamination is new and widespread (i.e., in at least two different cultures), discard all media, stock solutions, trypsin, and so forth in current use.
(5) If the contamination is widespread, multispecific, and repeated, check (a) the laboratory’s sterilization procedures (e.g., the temperatures of ovens and autoclaves, particularly in the center of the load, the duration of the sterilization cycle), (b) the packaging and storage practices, (e.g., unsealed glassware should be resterilized every 24 h), and (c) the integrity of the aseptic room and laminar-flow hood filters
(6) Do not attempt to decontaminate cultures unless they are irreplaceable.
Related Services
Endotoxin Detection and Removal Services
Mycoplasma Detection & Elimination
Sterility Testing
References:
- Langdon, Simon P. Cancer cell culture. Humana, 2010.
- Freshney, R. Ian. Culture of specific cell types. John Wiley & Sons, Inc., 2005.
- Staib, P.; et al. Prediction of individual response to chemo- therapy in patients with acute myeloid leukaemia using the chemosensitivity index Ci. Br. J. Haematol. 2005, 128, 783–91.
- Hay, R. J.; et al. Mycoplasma infection of cultured cells. Nature. 1989, 339.6224: 487-488.