Cytochrome P450 Inhibition Assay
- Service Details
- Features
- FAQ
- Explore Other Options
Cytochrome P450 (CYP450) is a superfamily of monooxygenases that are found predominantly in the liver. These enzymes are responsible for the metabolism of xenobiotics, with more than 80% of clinically used drugs being substrates of one or more CYP450 isoforms. CYP450 inhibition occurs when a drug, or its metabolite, blocks the metabolic activity of one or more of these enzymes. In cases where a given CYP450 isoform is responsible for the metabolism of another drug, inhibiting this enzyme may lead to a significant change in its metabolism, resulting in drug-drug interactions (DDIs).
There are two main types of CYP450 inhibition:
Reversible Inhibition: This is a time-dependent process in which the inhibitor reversibly binds to the enzyme, leading to the prevention of metabolism of other substrates. The most common types of reversible inhibition are competitive, non-competitive, and uncompetitive. Upon removal of the inhibitor, the enzyme activity is restored.
Irreversible Inhibition (Mechanism-Based Inactivation): This is a permanent process where the inhibitor is metabolized by the enzyme to form a highly reactive intermediate. This intermediate then binds covalently to the active site of the enzyme, rendering it inactive. This process is time-dependent and irreversible.
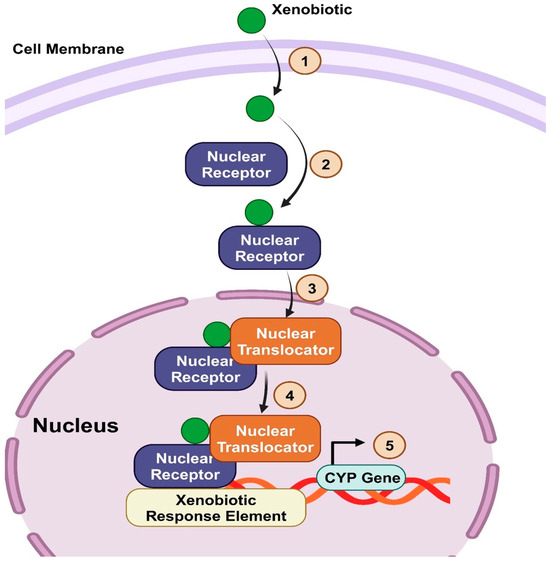 Fig. 1. Xenobiotics activate CYP450 gene expression (Hossam Abdelmonem B, Abdelaal NM, et al., 2024).
Fig. 1. Xenobiotics activate CYP450 gene expression (Hossam Abdelmonem B, Abdelaal NM, et al., 2024).
Why CYP450 inhibition assays?
- Assess potential drug-drug interactions (DDIs) early in drug development.
- Support regulatory submissions (FDA, EMA, etc.) by evaluating metabolic safety profiles.
- Optimize lead compounds by identifying inhibitory effects on key CYP isoforms.
IC₅₀ and Kᵢ values
IC₅₀ (half-maximal inhibitory concentration): The concentration of the test compound that results in 50% inhibition of enzyme activity. IC₅₀ values are commonly used for high-throughput screening of potential inhibitors.
Kᵢ (inhibition constant): A mechanistic parameter that describes the binding affinity of an inhibitor to the enzyme. Kᵢ values offer deeper insight into the type and strength of inhibition and are generally more predictive of in vivo interactions.
Creative Bioarray's Cytochrome P450 Inhibition Assay Service
Workflow
Step1: Study Design & Consultation
Tailored experimental setup based on compound properties, target isoforms, and regulatory requirements.
Step2: Sample Preparation
Microsomes, recombinant enzymes, or hepatocytes are selected and prepared for incubation.
Step 3: Assay Execution
IC₅₀ and/or Kᵢ determination performed with probe substrates using optimized incubation conditions.

Step 4: Metabolite Quantification
Probe metabolite formation measured by LC–MS/MS.
Step 5: Data Analysis & Interpretation
Calculation of IC₅₀, Kᵢ, and inhibition mode; results validated for quality.
Step 6: Reporting & Delivery
Comprehensive report including raw data, analysis, and scientific interpretation.
Protocol
| Parameters | Details |
| CYP Enzyme | CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4/5 (other isoforms are available on request) |
| Test System | Human liver microsomes, recombinant CYP enzymes or cryopreserved human hepatocytes |
| Sample Test Concentration | 8-10 different concentration gradients |
| Replicates | 3 (minimum) |
| Analytical Method | LC–MS/MS |
| Incubation Time | Typically, 5–30 minutes (optimized per CYP isoform and substrate) |
| Data Delivery | IC₅₀ and/or Kᵢ values |
We also offer CYP450 Time-Dependent Inhibition (TDI) assays to evaluate mechanism-based enzyme inactivation, complementing reversible inhibition studies for a more complete assessment of drug–drug interaction potential.
Features
Flexible Scale
Available for exploratory studies or large-scale validation.
High Throughput
Screening capability for multiple compounds and isoforms simultaneously.

Customizable Design
Assays tailored to client-specific study needs, including additional substrates, species differences, or time-dependent inhibition studies.
FAQ
Why should I measure both IC₅₀ and Kᵢ?
IC₅₀ can be a suitable metric for early ranking of compounds. Kᵢ is mechanistically informative and is required for an accurate assessment of DDI risk. We recommend that both parameters be determined for a more complete understanding of enzyme inhibition.
Can you evaluate time-dependent inhibition (TDI)?
Yes. In addition to reversible inhibition studies, we can offer TDI assays to assess mechanism-based inactivation of enzymes.
Which probe substrates do you use? Can they be customized?
We use FDA- and EMA-recommended probe substrates for standard CYP isoforms. Alternative substrates can be included based on project requirements.
Reference
- Hossam Abdelmonem B, Abdelaal NM, et al. Decoding the Role of CYP450 Enzymes in Metabolism and Disease: A Comprehensive Review. Biomedicines. 2024; 12(7):1467
Explore Other Options