Top 5 Pitfalls in In Vitro ADME Assays and How to Avoid Them

Why In Vitro ADME Data Often Misleads Decisions
If there's one topic that brings focus to a medicinal chemistry group, it's ADME. Used early and often during drug discovery, in vitro ADME assays are intended to help teams make decisions about which compounds to move forward with, which to optimize, and which to kill. Done right, ADME assays should decrease risk, save time and minimize chances of expensive late-stage attrition.
In practice, however, many ADME-related failures have little to do with the chemistry. Faulty assay design, application, or interpretation can lead teams astray despite generating the cleanest, most reproducible data imaginable. Dreams of promising molecules are shattered as teams kill programs too soon. Fatalities pile up in programs that continue forward with unjustified confidence.
The majority of these problems are caused by several common assay design pitfalls. Learn about them and how to avoid them so you can generate more useful, trustworthy ADME data that has a positive impact on your projects.
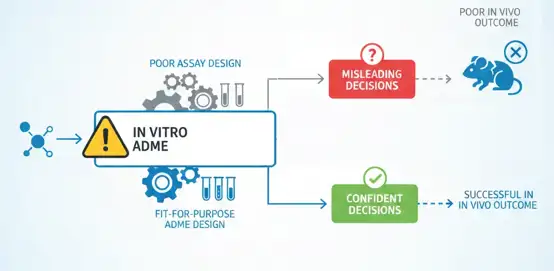
The 5 Critical Pitfalls
Pitfall 1: Ignoring the Intended In Vivo Context
What goes wrong:
One of the most common mistakes is running ADME assays without clearly defining how the drug is expected to work in vivo. Typical examples include:
- Using the same ADME workflow for oral and parenteral drugs
- Ignoring the target indication (CNS, oncology, or peripheral disease)
- Applying systemic exposure criteria to drugs intended for local or tissue-specific action
In many projects, ADME becomes a "standard checklist" rather than a context-driven strategy.
Why it matters:
When in vitro assays are disconnected from the real therapeutic goal, the resulting data can be misleading. This often leads to:
- Premature rejection of compounds that could be effective in the intended setting
- Over- or underestimation of pharmacokinetic (PK) risk
- Misaligned optimization efforts in medicinal chemistry
In short, the data may be correct, but the conclusions are not.
How to avoid it:
Before designing any ADME experiment, it is essential to answer a few basic questions:
- What is the intended route of administration?
- Where does the drug need to reach to be effective?
- Does it need to cross biological barriers such as the blood-brain barrier (BBB)?
Practical examples:
- CNS drugs: BBB permeability and brain clearance are often more critical than high systemic stability. A compound with moderate plasma clearance may still be viable if it achieves sufficient brain exposure.
- Locally acting drugs: For inhaled, topical, or gut-restricted therapies, systemic metabolic stability may be far less relevant than tissue retention or local concentration.
Real-world note:
Designing ADME assays around real in vivo needs turns data into insight, not noise.

Pitfall 2: Over-Reliance on a Single In Vitro Model
Common misconceptions
Another frequent issue is assuming that one in vitro model can represent a complex biological process. Typical examples include:
- Using only liver microsomes to assess metabolic stability
- Relying solely on Caco-2 assays to predict oral absorption
- Ignoring transporter effects or non-CYP metabolic pathways
While these models are valuable, they each capture only part of the picture.
The risks
Over-reliance on a single model can lead to:
- Large errors in predicting clearance and bioavailability
- Failure to identify drug-drug interaction (DDI) risks
- False confidence in compounds that later fail in vivo
Biological systems are redundant and interconnected. Single-model conclusions are often too simple.
A better approach
A more reliable strategy is to use complementary model combinations, such as:
- Liver microsomes + hepatocytes to capture both enzyme activity and cellular uptake
- Caco-2 + MDCK, with or without transporter inhibitors, to better understand permeability and efflux
- Additional transporter or enzyme-specific assays when red flags appear
Model complexity should also evolve with project stage. Early discovery may favor higher throughput and simpler systems, while lead optimization may justify more physiologically relevant models, such as 3D liver systems or organ-on-chip platforms.
The key is not to use every model available, but to use the right combination for the question at hand.
Pitfall 3: Improper Concentration Selection
Many in vitro ADME experiments are performed at concentrations that have little to do with real in vivo exposure. This often happens for convenience, sensitivity, or historical reasons.
Common problems
- Testing at excessively high concentrations, leading to:
- Non-specific binding
- Apparent metabolic saturation
- Ignoring compound solubility limits
- Failing to consider the true free (unbound) drug concentration
Why this matters
Poor concentration selection can cause:
- Over- or underestimation of metabolic stability
- False positives or negatives in transporter assays
- Data that look consistent but have no predictive value
In some cases, the compound is evaluated under conditions it will never experience in vivo.
How to design better concentration ranges
A more realistic approach includes:
- Designing concentrations based on expected in vivo exposure, not just assay convenience
- Evaluating and documenting:
- Solubility limits
- Plasma protein binding
- Using multiple concentrations rather than a single fixed value
- Interpreting results based on free drug concentration (fu) whenever possible
These steps add minimal complexity but significantly improve data relevance.
Pitfall 4: Neglecting Non-Specific Binding and Matrix Effects
What gets overlooked
In many ADME assays, a significant fraction of the compound never actually participates in the biological process being measured. Common sinks include:
- Adsorption to plastic labware
- Binding to extracellular matrix components
- Sequestration by microsomal or cellular membranes
- This issue is especially pronounced for lipophilic compounds.
Impact on data quality
If non-specific binding is ignored, the results may show:
- Artificially high apparent clearance
- Poor reproducibility between experiments
- Confusing discrepancies between in vitro and in vivo data
The assay measures what disappears-not what is metabolized.
Practical solutions
To address this issue:
- Assess plastic and microsomal binding during assay development
- Use low-binding consumables when appropriate
- Apply binding corrections in data analysis
- Clearly document correction methods in reports
Acknowledging binding effects does not weaken the data-it makes them more honest and interpretable.
Pitfall 5: Misinterpretation of In Vitro-In Vivo Correlation (IVIVC)
The fundamental misunderstanding
A common mistake is treating in vitro ADME results as direct predictors of in vivo behavior. Examples include:
- Assuming in vitro clearance equals in vivo clearance
- Ignoring blood-flow limitations
- Overlooking tissue distribution and species differences
This often leads to overconfidence in early-stage predictions.
The right perspective
In vitro ADME assays are risk-screening tools, not final answers. They are most powerful when used for:
- Ranking compounds relative to each other
- Identifying mechanisms of clearance or transport
- Highlighting potential liabilities early
They are far less reliable as standalone predictors of absolute PK parameters.
How to use IVIVC more effectively
- Continuously compare in vitro data with emerging in vivo PK results
- Build internal project-specific IVIVC experience rather than relying on generic assumptions
- Use modeling tools, such as PBPK, to integrate in vitro data with physiological context
Used correctly, IVIVC becomes a learning process, not a pass-fail test.
Quick Self-Check: Is Your ADME Strategy Fit for Purpose?
- Does each assay reflect the intended in vivo scenario?
- Are multiple complementary models used where needed?
- Are test concentrations physiologically relevant?
- Have non-specific binding and matrix effects been addressed?
- Are in vitro results interpreted as screening tools rather than predictions?
If you answered "no" to any of these, your ADME data may be misleading.
How We Help
We offer customized in vitro ADME assay design, integrating:
- Multi-model approaches tailored to your compounds
- Data interpretation and project-specific guidance
- Support for decision-making, not just data generation
The right ADME strategy helps you identify promising candidates faster, reduce unnecessary experiments, and make confident, data-driven decisions.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
|---|---|
| Safety Evaluation Services | Creative Bioarray stands as a premier provider of comprehensive safety evaluation studies, adeptly serving a diverse spectrum of industries. Our expertise extends to a broad range of product categories, encompassing pharmaceuticals, cosmetics, and personal care items within the realm of daily chemical products, as well as disinfectants and food products |
| Drug Metabolism and Pharmacokinetics (DMPK) | Creative Bioarray provide comprehensive in vitro and in vivo DMPK solutions to support lead optimization, candidate selection, and regulatory submissions, accelerating the path from discovery to clinical development |