Bioavailability and Bioequivalence
- Service Details
- Features
- FAQ
- Explore Other Options
Pharmaceutical manufacturers are required to conduct BA/BE studies to support NDAs and ANDAs or post approval changes in formulation, manufacturing process and scale-up, as per regulatory agencies' guidelines globally. Creative Bioarray offers expert pharmacokinetic (PK) testing services to support post-approval formulation or manufacturing changes and to assess bioavailability and bioequivalence in compliance with global regulatory standards.
What are Bioavailability (BA) and Bioequivalence (BE)?
BA is defined as the rate and extent of appearance of the active drug substance or active moiety in the body circulation, or the rate and extent to which it becomes available at the site of drug action.
Absolute BA (extravascular versus intravenous administration) and Relative BA (two different formulations) in preclinical research are critical in selecting the optimal dosage form and route for IND enabling studies.
BE is established when two pharmaceutical products (typically a Test formulation and a Reference Listed Drug, or RLD) contain the same active ingredient and are absorbed at the same rate and extent, resulting in comparable concentration-time profiles in the systemic circulation.
This is a regulatory requirement for generic drug approval (ANDA) or for bridging various dosage forms and different manufacturing changes in NDA submissions.
Our Capabilities
We offer flexible, high-resolution BA/BE services specifically designed to support the transition from discovery to IND-enabling studies.
Species Expertise:
- Rodents: Mice, Rats, Guinea pigs
- Non-rodents: Dogs, Minipigs, Non-human primates
Drug Formulation & Routes
- Formulations: Solutions, Suspensions, Tablets, Capsules, Powders, Emulsions, Liposomes, and other novel delivery systems.
- Routes: Oral (PO), Intravenous (IV), Subcutaneous (SC), Intramuscular (IM), Intraperitoneal (IP), Topical, and more.
Bioanalytical & Pharmacokinetic Expertise
- Triple-quadrupole and high-resolution LC-MS/MS for quantitation down to low pg/mL as required.
- UPLC systems, automated sample prep (SPE, protein precipitation), and validated bioanalytical workflows.
- Ligand binding assays (ELISA, MSD) for biologics; capability for immunogenicity (anti-drug antibody) screening.
Study Design
- Bioavailability
- Bioequivalence
Bioavailability
The study is typically divided into different groups based on the selected dosing methods, with the most widely used intravenous (iv) injection and oral administration (po). A standard study investigating the oral BA of a drug compound in rats is as follows.
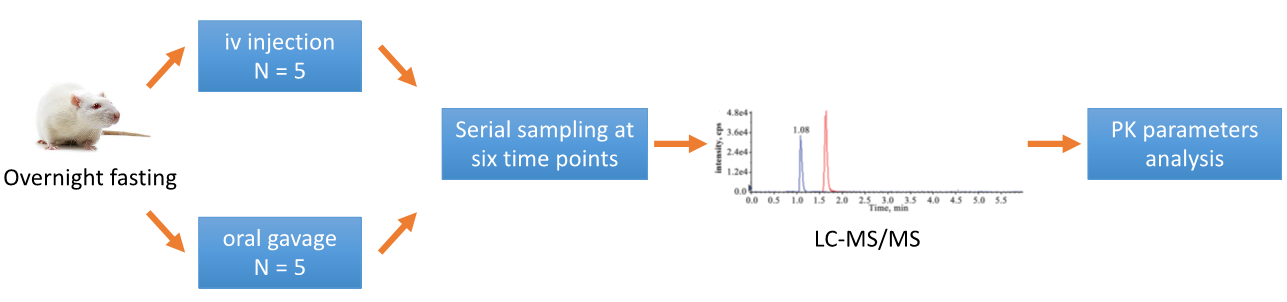 Fig. 1. Standard study investigation of oral BA of a drug.
Fig. 1. Standard study investigation of oral BA of a drug.
Bioequivalence
A conventional BE study design is a single-dose, two-treatment, crossover, in vivo experiment with pharmacokinetic (PK) endpoints. A typical BE study investigating the oral BE of two drug formulations in rats is as follows.
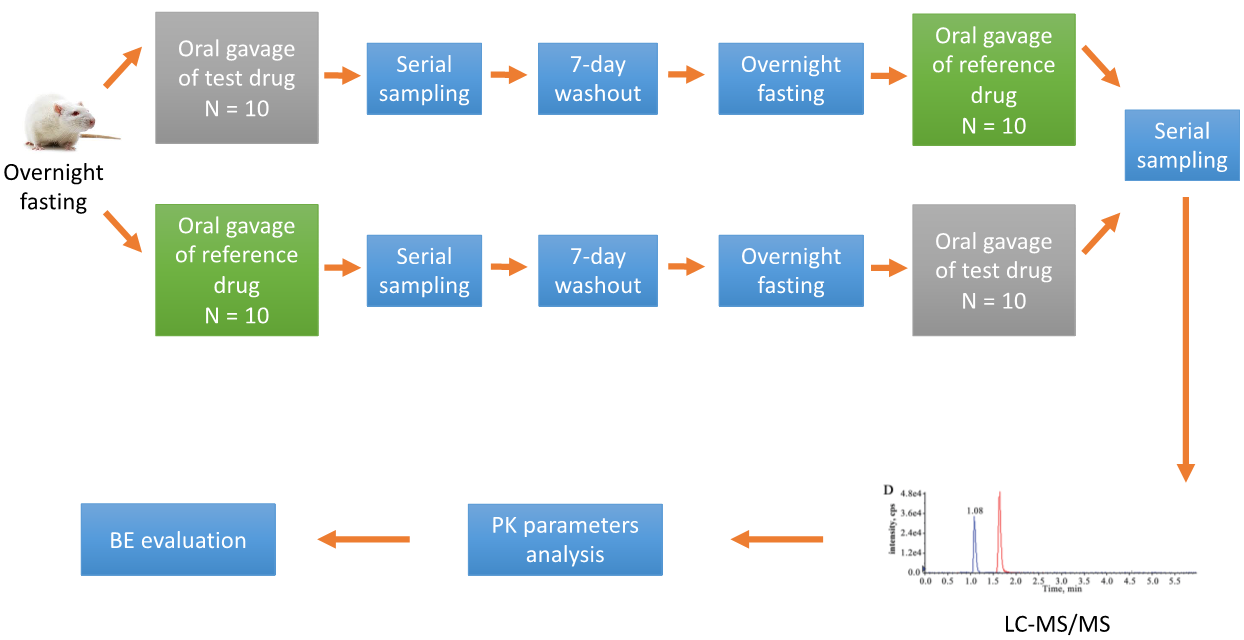 Fig. 2. Standard study investigation of the oral BE of two drug formulations.
Fig. 2. Standard study investigation of the oral BE of two drug formulations.
According to different research purposes, our experimental design can be adjusted in the following aspects, such as
- Additional animals
- Custom timepoints
- Parallel non-crossover designs
- Higher-order crossover designs
- Fed/food effects
- Sample size calculation
Why Partner with Us?
High-sensitivity bioanalysis
State-of-the-art LC-MS/MS and LBA platforms, enabling robust PK characterization for low-exposure modalities.

Accelerated Project Timelines
Leveraging optimized SOPs and automated bioanalytical workflows, we significantly reduce turnaround times (TAT) for BE/BA analysis without compromising data quality, saving critical time in your drug development program.

Customized Solutions
We offer tailored study designs to meet the specific needs of each project, from simple bioavailability assessments to complex bioequivalence studies for high-variability drugs.
Experienced Team
Our team of experts has extensive experience in preclinical pharmacokinetics and bioanalysis, ensuring reliable and accurate results.
FAQ
When are BA/BE studies required during drug development?
BA studies are important during lead optimization and preclinical development to screen for candidates with acceptable PK profiles. BE studies are required for ANDA submissions for generics, as well as any major change to the formulation, manufacturing site, or process of an already approved product.
What is the usual BE acceptance range?
Regulators require a 90% confidence interval for the geometric mean ratio to remain between 80.00–125.00% for both AUC and Cmax for most small-molecule immediate-release products unless product-specific documentation indicates different standards. Some study designs or tighter acceptance limits may be used for narrow-therapeutic-index drugs.
What are the key parameters measured in bioavailability and bioequivalence studies?
The main PK parameters of interest are the area under the concentration-time curve (AUC), maximum plasma concentration (Cmax), time to maximum concentration (Tmax), and sometimes terminal half-life (t1/2).
Do you run BE studies for biologics / complex modalities?
Yes—BE for biologics frequently relies on comparative PK/PD, immunogenicity assessment, and functional assays rather than classic small-molecule metrics. We offer ligand-binding assays, PD biomarker analysis, and study designs tailored for large molecules.
What is the main difference between absolute and relative bioavailability?
Absolute bioavailability is the comparison of systemic availability after extravascular administration (e.g. oral) to after intravenous administration (100% bioavailable). Relative bioavailability is the comparison of bioavailability between two different formulations of the same drug, administered by the same route.
Explore Other Options