Stem Cell Therapy

Stem cell therapy is rapidly advancing, attracting growing attention for its potential to treat diverse diseases. With their unique ability to self-renew and differentiate into various cell types, stem cells offer powerful possibilities for regenerating damaged tissues and organs, showing significant potential in treating neurological, cardiovascular, and certain cancerous conditions.
With the global entry into a phase of rapid industrialization of cell therapy products, GMP-grade cytokines with regulatory functions in stem cell differentiation and immune cell activation, are seeing their quality directly impacting the efficacy and safety of the final products. Creative Bioarray, has identified the demand and committed to providing high-quality GMP-grade cytokine products. These products can be used to support cell culture expansion and functional regulation of stem cells. Our products undergo stringent quality control to meet the high standards required for various research and applications.
Why Choose Our GMP Products?
High-quality cytokines are critical to ensuring the safety, efficacy, and consistency of cell therapy products. Our GMP-grade cytokines are manufactured under validated processes and stringent quality systems to meet the highest industry and regulatory standards:
- Batch-to-Batch Consistency: Each production lot is subject to comprehensive testing to guarantee reproducible performance and reliable results.
- High Purity and Bioactivity: Cytokines are produced with exceptional purity and verified biological activity to ensure optimal cell growth and functionality.
- Process Robustness: Scalable and well-controlled production ensures stable supply and consistent product quality across large batches.
- Endotoxin and Contaminant Control: Stringent QC testing minimizes the risk of endotoxin, mycoplasma, or adventitious agent contamination.
Cytokines Designed for Stem Cell Culture
Embryonic / Pluripotent Stem Cells
Embryonic / pluripotent stem cells can differentiate into various cell types of the endoderm, mesoderm, and ectoderm. They possess key advantages such as self-renewal and the ability to generate diverse cell types, making them foundational cell sources for stem cell–based therapies.
Through the controlled addition of specific cytokines, these stem cells can be directed to differentiate into targeted cell lineages. For example, treatment with retinoic acid and nerve growth factor (NGF) can induce differentiation into neural cells, while bone morphogenetic protein (BMP) promotes the formation of osteoblasts. This capacity for directed differentiation enables their application in treating a wide range of conditions, including nerve injuries and bone fractures.
GMP-grade Cytokines for Embryonic / pluripotent Stem Cells
| Activin A | BMP 2 | EGF | FLT3 Ligand |
| FGF 4 | BMP 4 | EPO | IL-15 |
| IL-2 | IL-6 | IL-7 | Noggin |
| Wnt3A | FGF-7 | HGF | IGF-1 |
| SCF | TGF-beta | FGF-10 | PDGF-BB |
| VGEF |
Neural Stem Cells
Neural stem cells (NSCs) are self-renewing progenitor cells capable of generating neurons, astrocytes, and oligodendrocytes through asymmetric division. These cells serve as the fundamental source for neural tissue formation and regeneration. NSCs are primarily found in the embryonic neural tube and in specific brain regions, including the subventricular zone and the dentate gyrus of the hippocampus.
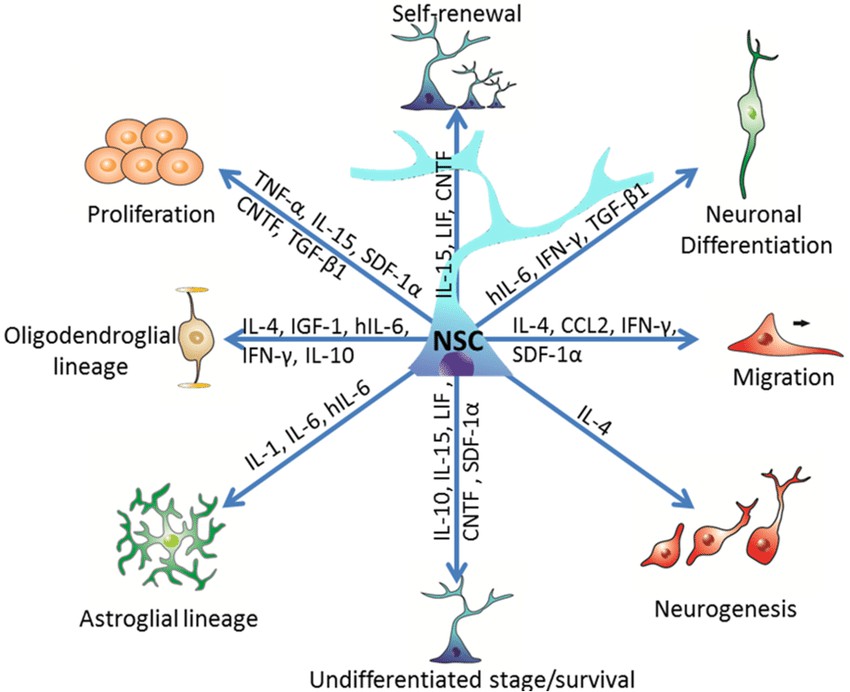 Fig. 1. Effects of immunological cytokines on neural stem cells
(Gutierrez-Fernandez F, Pinto-Gonzalez M, et al., 2014).
Fig. 1. Effects of immunological cytokines on neural stem cells
(Gutierrez-Fernandez F, Pinto-Gonzalez M, et al., 2014).
Neural stem cells play an important role in neural development and repair of damaged neural tissue. Transplantation of neural stem cells can repair and replace damaged brain tissue and can partially reconstruct the circuits and function of the brain. In addition, neural stem cells can also be used as gene carriers for gene therapy of intracranial tumors and other diseases.
GMP-grade Cytokines for Neural Stem Cells
| Noggin | PDGF-BB | TGF beta | IGF-1 |
| BMP-2 | EGF | FGF-8b | IL-3 |
| BMP-4 | EPO | GDNF | IL-6 |
| BNDF | FGF-4 | GM-CSF | LIF |
| VEGF | SCF |
Mesenchymal Stem Cells
Mesenchymal stem cells are pluripotent stem cells derived from immature embryonic connective tissue and are mesoderm pluripotent stem cells. They are mainly located in the connective tissue and the stroma of organs. Bone marrow is the most abundant source. Mesenchymal stem cells have the ability of self-renewal and can differentiate into osteoblasts, chondrocytes, adipocytes, myocytes and other cell types. In addition, they also have immunomodulatory and anti-inflammatory effects as well as tissue repair and regeneration effects.
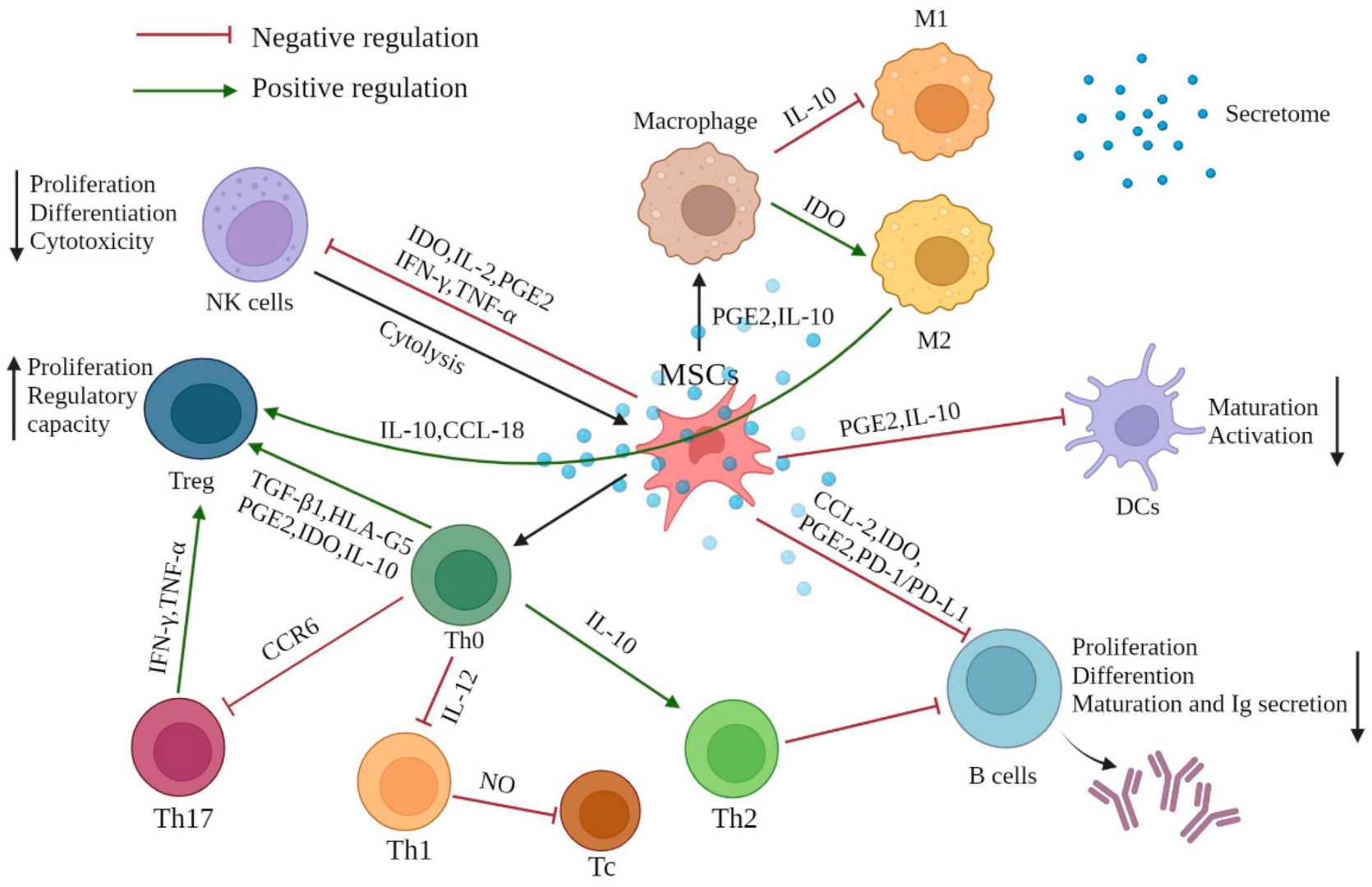 Fig. 2. The effect of MSC-mediated immunomodulation on immune cells (Huang Y,
Wu Q, et al., 2022).
Fig. 2. The effect of MSC-mediated immunomodulation on immune cells (Huang Y,
Wu Q, et al., 2022).
Mesenchymal stem cells have broad applications in tissue repair and regenerative medicine and are used to treat diseases such as osteoarticular, neurological, and cardiovascular diseases. They promote tissue repair and functional recovery by differentiating into specific cell types and secreting growth factors.
GMP-grade Cytokines for Mesenchymal Stem Cells
| IL-3 | FLT3 Ligand | PDGF-BB | BMP-6 |
| LIF | G-CSF | SCF | FGF-4 |
| BMP-2 | GM-CSF | TGF-beta1 | IGF-1 |
| BMP-4 | IL-2 | VEGF | IL-6 |
| EGF | IL-6 | TGF-beta3 | EPO |
Hematopoietic Stem Cells
Hematopoietic stem cells (HSCs) are the origin of all blood and immune cells. They possess remarkable self-renewal, multilineage differentiation, and homing abilities, enabling them to generate red blood cells, white blood cells, and platelets. Often called "universal cells," HSCs are regarded as the ancestral stem cells that sustain the body's hematopoietic and immune systems.
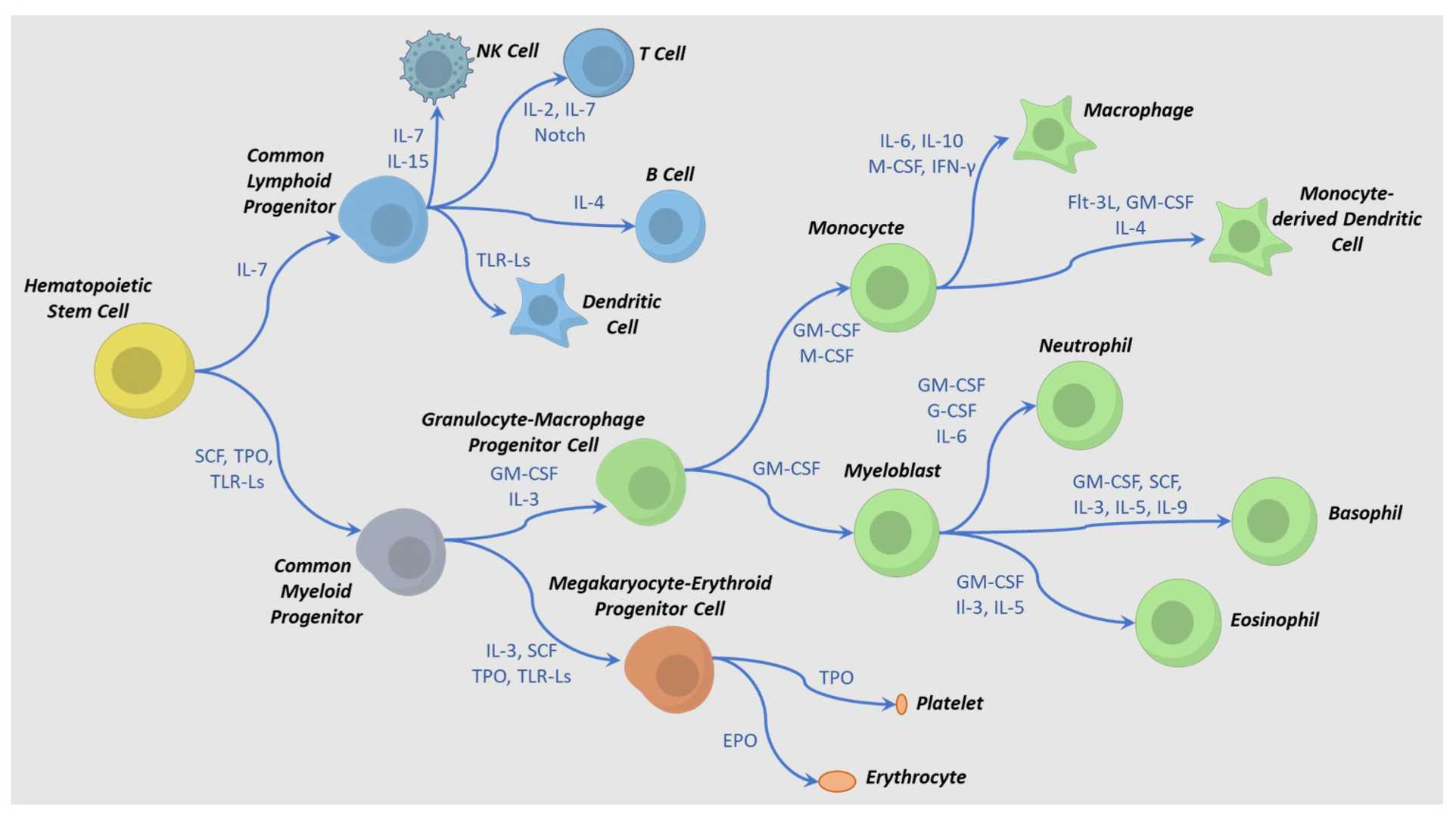 Fig. 3. Cytokine-directed differentiation pathways for hematopoietic cell
lineages (Kao C-Y, Mills JA, et al., 2023).
Fig. 3. Cytokine-directed differentiation pathways for hematopoietic cell
lineages (Kao C-Y, Mills JA, et al., 2023).
Hematopoietic stem cell transplantation is widely used in clinical settings for treating hematological malignancies such as leukemia, aplastic anemia, and myelodysplastic syndromes. This transplantation significantly increases the cure rates for these diseases.
GMP-grade Cytokines for Hematopoietic Stem Cells
| IL-3 | FLT3 Ligand | IL-2 | M-CSF |
| wnt-3a | G-CSF | IL-21 | SCF |
| BMP-2 | GM-CSF | IL-4 | TGF beta |
| BMP-4 | IL-10 | IL-6 | TPO |
| CM-CSF | IL-11 | IL-7 | VEGF |
| EPO | IL-15 | LIF |
Our Cytokines' Features
High Purity
Advanced purification technologies ensure cytokine purity surpasses 95%, minimizing impurities' impact on cells.
High Biological Activity
Stringent activity testing guarantees high biological efficacy of cytokines.
Free from Animal Origin
Ensures compatibility and safety.
High Batch Consistency
Strict production processes and quality control ensure high consistency across different batches.
GMP Manufacturing and Quality Control System
Production Capability
- GMP Facility: Equipped with GMP-compliant facilities and manufacturing sites approved by FDA and EMA for environment (clean room) control to be sterile and particulate-free.
- Production Equipment: Multi-functional bioreactors for cell culture and high-speed filling lines capable of customizing equipment according to specific needs from trial to commercial-scale manufacturing.
- Operational Standards: All production processes strictly follow GMP SOPs to ensure safe and consistent operation.
Comprehensive Quality Control System
- Compliance with International Standards: Manufactured in accordance with GMP guidelines and relevant pharmacopeial standards to ensure safety, purity, and consistency of cytokines and growth factors for cell therapy applications.
- End-to-End Quality Control: Maintains meticulous quality control records for raw material quality audit, in-process quality controls, and regular calibration and maintenance of production equipment.
Product Testing System
Every batch of products undergoes multiple testing measures to ensure they meet all necessary standards for clinical application:
- Identity and Purity Testing: Employs techniques like high-performance liquid chromatography (HPLC) and mass spectrometry (MS) to verify the identity and purity of cytokines and other bioactive substances, ensuring compliance with standards.
- Biological Activity Testing: Methods such as cell proliferation and migration assays evaluate the biological activity of cytokines and other bioactive substances, confirming their efficacy in cell therapy.
- Protein Content Testing: Accurate protein quantification using techniques such as enzyme-linked immunosorbent assay (ELISA) and Bradford method, ensuring concentration requirements.
- Safety Testing: Includes microbiological, endotoxin, and sterility testing to ensure that products are free from pathogens and contaminants before use.
References
- Gutierrez-Fernandez F, Pinto-Gonzalez M, et al. Neuro-immune interactions in the postnatal ventricular-subventricular zone. J Stem Cells. 2014. 9(1):53-64.
- Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells. 2014. 6(5):526-39.
- Kao C-Y, Mills JA, et al. Role of Cytokines and Growth Factors in the Manufacturing of iPSC-Derived Allogeneic Cell Therapy Products. Biology. 2023. 12(5):677.
Explore Other Options