P-gp & BCRP Inhibition Assay
- Overview
- Services
- Features
- FAQ
- Explore Other Options
P-glycoprotein (P-gp, MDR1, ABCB1) and Breast Cancer Resistance Protein (BCRP, ABCG2) are members of the ATP-binding cassette (ABC) transporter family, and are known to be involved in the absorption, distribution and elimination of drugs.
- P-gp is widely expressed in tissues such as the small intestine, liver, kidney, and blood–brain barrier, where it actively transports structurally diverse substrates out of cells, thereby modulating oral bioavailability and CNS penetration.
- BCRP is also expressed in the intestine, placenta, blood–brain barrier and liver, among other tissues, where it limits the absorption of various compounds and promotes their elimination. Modulation of BCRP activity can significantly impact drug bioavailability and safety.
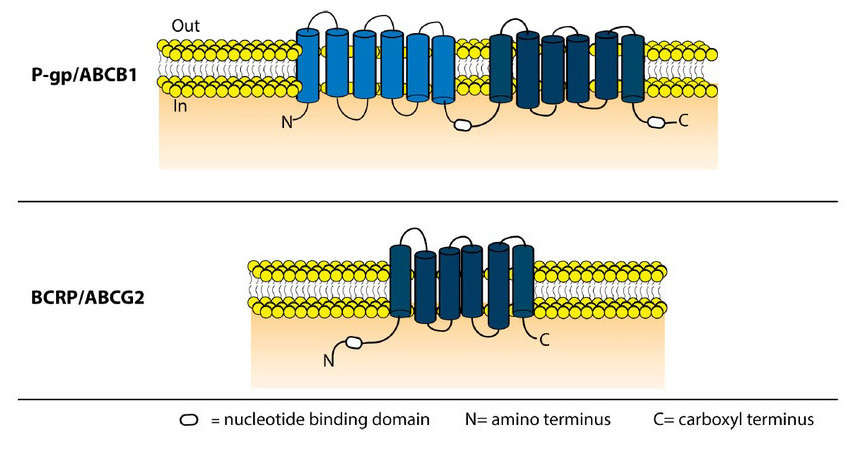 Fig. 1. Schematic representation of the domain structure of P-gp and BCRP (Jaramillo A C, Saig F A, et al., 2018).
Fig. 1. Schematic representation of the domain structure of P-gp and BCRP (Jaramillo A C, Saig F A, et al., 2018).
Understanding the interaction of drug candidates with P-gp and BCRP is essential for assessing their ADME (Absorption, Distribution, Metabolism, Excretion) properties and potential DDIs. Using physiologically relevant in vitro models (such as Caco-2, transfected cell lines and vesicle assays), our P-gp and BCRP Inhibition Assay Services provide regulatory-compliant data, helping you make informed decisions in drug development.
Service Offers
P-gp and BCRP transwell assays
Using human cell lines overexpressing P-gp or BCRP, the Transwell system is employed to evaluate the impact of test compounds on transporter-mediated substrate translocation across cell monolayers.
| Workflow |
|
| Test systems | Polarized cells grown on transwell plates
|
| Advantages | Physiologically relevant models that provide insights into both passive permeability and transporter-mediated inhibition. |
P-gp and BCRP vesicle assays
Membrane vesicles containing human P-gp or BCRP are used to measure ATP-dependent substrate uptake or efflux, directly reflecting transporter activity.
| Workflow |
|
| Test systems | Inverted plasma membrane vesicles, from cells overexpressing transporter |
| Advantages | Simple and highly specific assay, minimizing interference from cellular metabolism or other transport pathways. |
Features
Flexible Models
We offer various cell lines (wild-type/transfected) and vesicle systems to suit your research needs.

High Sensitivity
LC-MS/MS detection ensures accurate quantification.

Customizable
Substrate/inhibitor selection tailored to client needs.
Rapid Turnaround
Streamlined protocols with fast reporting.

Customizable Reports
Comprehensive reports including methodologies, raw data, and data interpretation, supporting regulatory submissions and scientific publications.
FAQ
Why is P-gp/BCRP inhibition testing important?
P-gp and BCRP are critical efflux transporters that affect the absorption, distribution, and elimination of drugs and other substrates. Their inhibition can result in increased systemic exposure, enhanced brain penetration, and clinically significant drug–drug interactions (DDIs). Inhibition of P-gp/BCRP can have a major impact on the pharmacokinetics and pharmacodynamics of a drug candidate, thus the need for inhibition testing to understand the implications on efficacy and safety in drug development.
Which cell lines do you use?
We employ well-established cell models tailored to different applications, including:
Caco-2 cells, which endogenously express P-gp, serve as a physiologically relevant model of the intestinal epithelium.
MDCK-II cells overexpressing human P-gp or BCRP (ideal for transporter-specific assays with high throughput).
Can you provide drug–drug interaction (DDI) predictions?
Yes. By measuring IC50 or Ki values of the test compounds and comparing them with the clinically relevant unbound plasma concentrations, we can predict transporter-mediated DDIs, providing valuable insights into potential safety risks and supporting regulatory submissions.
What types of compounds can be tested?
Our assay is designed to be compatible with a wide variety of compounds including small-molecule drugs, natural products, prodrugs, metabolites and even certain biologics. Special study design is available for compounds with specific physicochemical properties.
How is inhibition assessed?
Inhibition of substrate transport or uptake is determined in the presence and absence of test compounds. The extent of inhibition is quantified by calculating the percent inhibition. The concentration–response data can be further analyzed to calculate IC50 or Ki values.
Explore Other Options