What Are CAR T Cells?
CAR-T Cells (Chimeric Antigen Receptor T-Cell Immunotherapy) is a type of cancer immunotherapy that genetically modifies a patient's own T cells to express chimeric antigen receptors (CARs) on their surface, which enables the T cells to specifically recognize and target tumor cells for destruction.
Fundamental Concepts of CAR-T Cell Technology
T cells
T cells are a subset of lymphocytes, which are the main cells of the adaptive immune system, and they can be classified into two main subsets: CD4+ helper T cells and CD8+ cytotoxic T cells. CD8+ T cells have the ability to directly kill target cells through the secretion of cytotoxic molecules such as perforin and granzymes. In CAR-T cell therapy, CD8+ T cells are commonly used as the effector cell population due to their robust cytotoxic potential.
Chimeric antigen receptors (CARs)
CARs are artificially constructed fusion proteins, consisting of three main domains: the extracellular domain, transmembrane domain, and intracellular signaling domain.
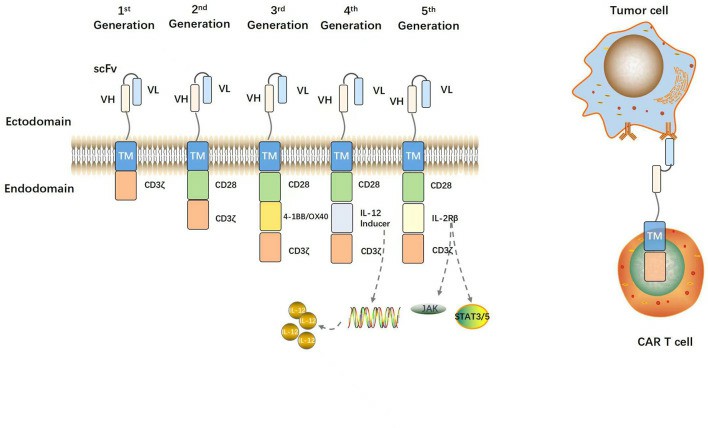 Fig. 1. Structural evolution of CARs (Geng P, Chi Y, et al., 2023).
Fig. 1. Structural evolution of CARs (Geng P, Chi Y, et al., 2023).
1. Extracellular Domain
This domain is critical for antigen recognition and includes two key substructures:
- Antigen-Binding Domain:
Most commonly formed by a single-chain variable fragment (scFv), which is derived from the variable regions of a monoclonal antibody's heavy (VH) and light (VL) chains connected by a short peptide linker. The scFv preserves the specificity and affinity of the parent antibody.
Function: Provides CAR-T cells with the ability to bind to specific tumor antigens. Unlike conventional T cell receptors (TCRs), scFvs recognize antigens in an MHC-independent manner. This enables recognition of non-peptide antigens and allows CAR-T cells to overcome tumor immune evasion strategies such as MHC downregulation.
Development Status: CD19, CD20, CD22, CD30, CD33, BCMA, etc., are commonly targeted antigens, while targeting tumor-associated antigens (TAAs) may lead to on-target/off-tumor toxicity. Efforts have been made to develop dual-target CARs (TanCAR, loop CAR) and logic-gated CARs (AND-gate CARs) to improve specificity.
- Hinge Domain:
Typically derived from the hinge regions of IgG or extracellular portions of CD8α or CD28 (e.g., IgG1, IgG4).
Function: Connects the scFv to the transmembrane domain, providing spatial flexibility so the scFv can access antigenic epitopes on the tumor surface. Optimizing hinge length helps adjust the distance between CAR-T cells and target cells, improving binding efficiency.
Research Progress: Different epitope locations require different hinge lengths to overcome steric hindrance and enhance binding.
2. Transmembrane Domain
This region anchors the CAR on the T cell membrane and connects the extracellular domain to the intracellular signaling components.
3. Intracellular Domain
Responsible for T cell activation and proliferation, it comprises two primary structures:
- Costimulatory Domain:
Derived from the Ig superfamily (e.g., CD28, ICOS) or TNF receptor superfamily (e.g., 4-1BB, OX40, CD27).
Function: Provides costimulatory signals that enhance T cell activation, proliferation, and anti-tumor response. For instance, CD28 enhances differentiation into effector T cells, while 4-1BB promotes mitochondrial biogenesis and fatty acid oxidation, supporting T cell persistence.
Status: Research is ongoing to identify additional molecules such as CD40, HVEM, GITR, and TLR2 to further improve CAR-T function.
- Signaling Domain:
Consists of TCR/CD3ζ or Fc receptor components, such as FcεRIγ, and typically contains immunoreceptor tyrosine-based activation motifs (ITAMs).
Function: Initiates downstream signaling cascades, leading to the activation of cytotoxic functions. CARs with a single ITAM have shown improved in vivo performance over those with multiple ITAMs and also promote the generation of central memory T cells, enhancing their longevity.
Status: Optimization of ITAM number and placement is being actively researched to achieve an optimal balance between activation strength and T cell persistence.
Mechanism of Action of CAR-T Cells
The therapeutic effect of CAR-T cells involves several sequential steps:
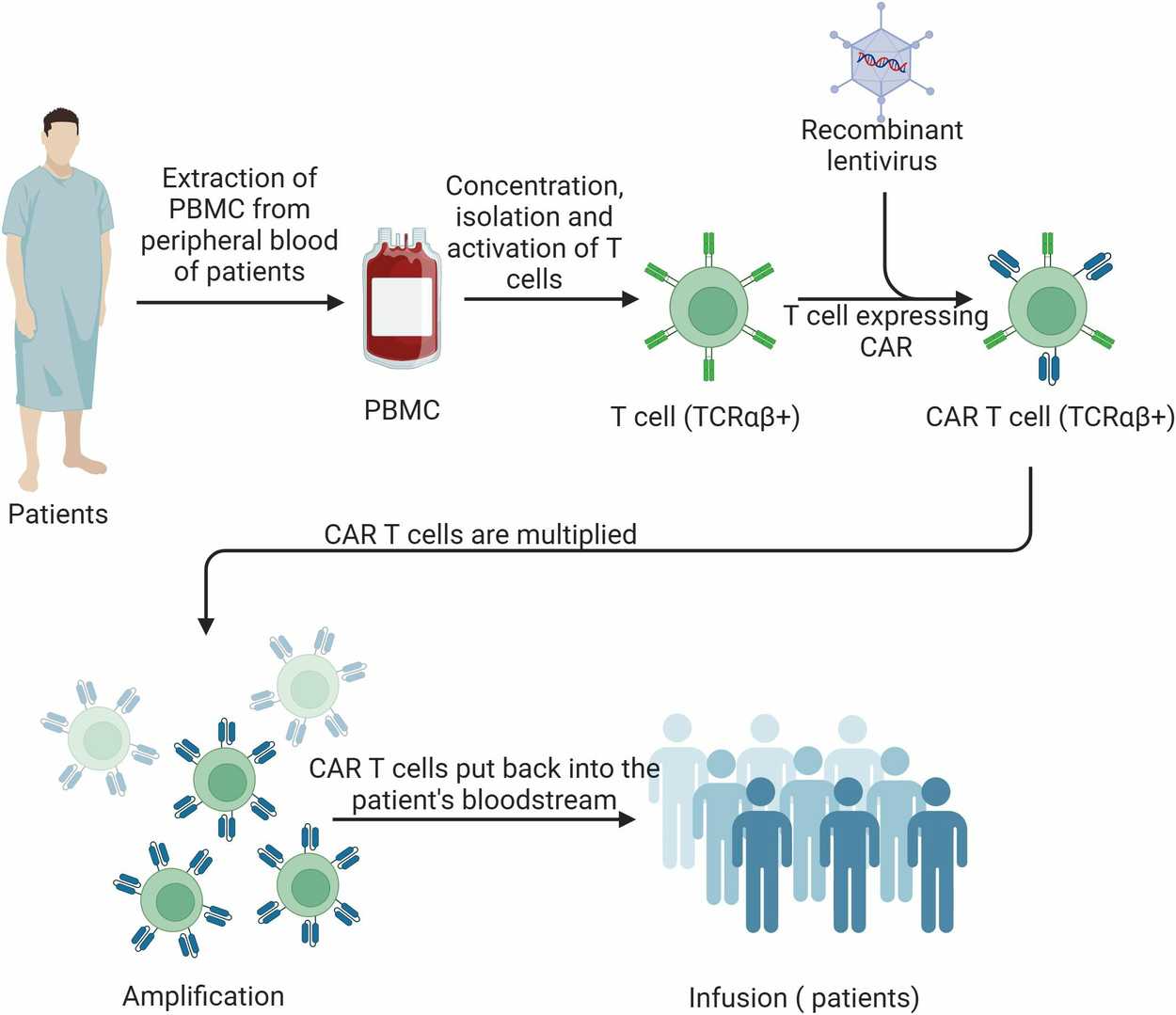 Fig. 2. Schematic of CAR-T cell therapy process (Kong Y, Li J, et al., 2025).
Fig. 2. Schematic of CAR-T cell therapy process (Kong Y, Li J, et al., 2025).
1. Antigen Recognition
CAR-T cells recognize tumor-specific antigens on the surface of cancer cells via their engineered CARs. The extracellular domain (scFv) of the CAR binds selectively to targets such as CD19 or HER2. This antigen-binding step is the prerequisite for CAR-T cell activation.
2. T Cell Activation
Upon antigen binding, the intracellular signaling domain of the CAR transmits activation signals. These signals, usually via CD3ζ and co-stimulatory motifs like CD28 or 4-1BB, activate key pathways (e.g., ITAM signaling) that stimulate T cell proliferation and effector functions.
3. Cytotoxic Effects
Once activated, CAR-T cells exert anti-tumor effects through multiple mechanisms:
- Direct Cytotoxicity: Release of perforin and granzymes leads to tumor cell lysis and apoptosis.
- Cytokine Secretion: Production of cytokines such as IFN-γ and TNF-α enhances the immune response and recruits other immune cells, including natural killer (NK) cells and macrophages.
- Immune Memory Formation: CAR-T cells can form long-lived memory cells, enabling rapid response upon tumor relapse and providing durable anti-cancer protection.
4. Ex Vivo Expansion and Reinfusion
After genetic modification in the laboratory, CAR-T cells are expanded in vitro using cytokines like IL-2. Once sufficient numbers are reached, they are infused back into the patient intravenously, where they circulate and seek out tumor cells expressing the target antigen.
5. Synergistic Immune Activation
In addition to direct tumor killing, CAR-T cells can activate and recruit other immune cell types, forming an immune synergy that amplifies the overall anti-tumor response.
6. Persistence and Memory
CAR-T cells exhibit long-term survival in vivo and can differentiate into memory T cells, allowing them to maintain surveillance and attack recurrent tumor cells, ensuring prolonged therapeutic benefit.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
| CAR-T Preclinical Characterization in vivo | CAR-T preclinical characterization is a challenging but necessary step before CAR-T medical products approval. Creative Bioarray provides "one-stop" service for your scientific research, comprehensive and reliable assay results are guaranteed. |
References
- Geng P, Chi Y, et al. Novel chimeric antigen receptor T cell-based immunotherapy: a perspective for triple-negative breast cancer. Front Cell Dev Biol. 2023. 29;11:1158539
- Kong Y, Li J, et al. CAR-T cell therapy: developments, challenges and expanded applications from cancer to autoimmunity. Front Immunol. 2025. 9;15:1519671.