Quantitative Tissue Distribution
Drug concentration measurements in target and non-target tissues have been thought to be crucial for predicting drug efficacy and safety. However, extrapolation based on plasma pharmacokinetics (PK) data often fails to depict tissue exposure accurately. The quantitative tissue distribution study provides a time course of radiolabeled drug distribution and in situ concentrations in various tissues and organs via quantitative whole-body autoradiography (QWBA). In brief, animals are dosed with radiolabeled drugs, and whole-body sections are collected at successive time points for high-resolution phosphor imaging of total radioactivity.
Creative Bioarray provides quantitative tissue distribution services to help our customers visualize true tissue distribution, facilitate tissue PK analysis and dosimetry prediction before the initiation of human mass balance studies.
Animal Species
- Rodents
- Non-rodents
Mice, Rats, Guinea pigs
Dogs, Minipigs, Non-human primates
Study Design
Animals are randomly divided into different groups based on their assigned time point of euthanasia. Following is a standard study investigating the tissue distribution of a [14C]-labeled drug and related material in rats.
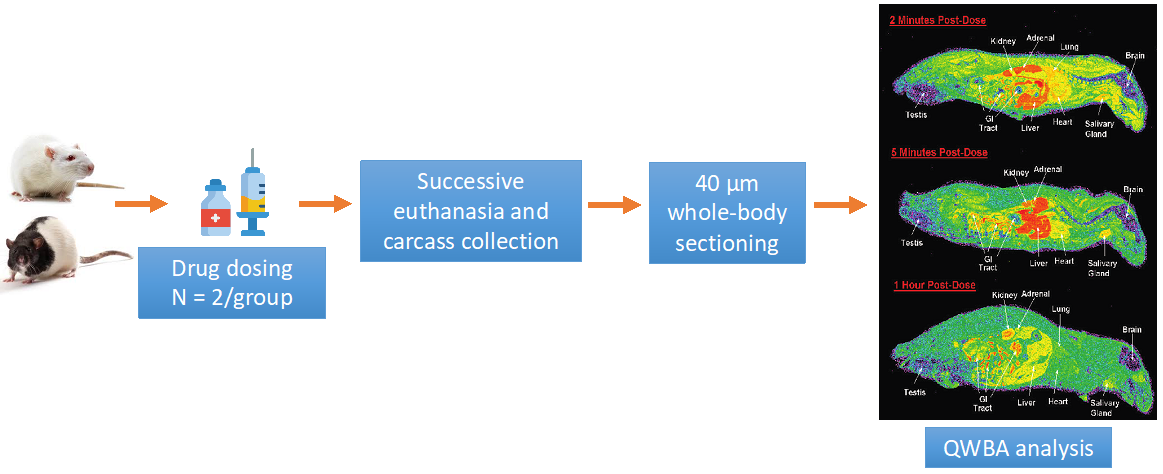 Figure 1. Standard study investigation of quantitative tissue distribution assay
Figure 1. Standard study investigation of quantitative tissue distribution assay
According to different research purposes, our experimental design can be adjusted in the following aspects, such as
- Additional animals
- Additional or custom time points
- Terminal blood plasma radioactivity quantification
- Placental transfer studies using pregnant animals
- Tumor penetration studies
- Gender effects
- Disease models
- Wet tissue radioactivity measurement in satellite animals
- Fed/food effects
- Multiple-dose studies
- Drug-drug interaction studies
- Nonradiolabeled sodium-iodide administration before 125I dosing
- Intracellular analysis via microautoradiography (MARG)
Drug dosing routes
- Default: intravenous (iv) and oral administration (po)
- Others: intraperitoneal (ip), intramuscular (im), and subcutaneous (sc) injections, iv cannulation
Dosing methods
The amount of radioactive dose is determined by the PK properties of the parent compound, typically under 100 µCi/kg. The radiolabeled drug is diluted with non radiolabeled compound to achieve the total targeted dose. While a single dose at the provided dosage is usually given to each animal, other drug dosing methods can be included to accommodate your specific needs.
- Additional dosage groups
- Repeated dosing for steady-state measurements
- Vehicle dosing
Sample collection
Sample collection typically lasts up to a week in a single-dose study but can be extended or shorten depending on the drug's half-life. A sample period covering ten plasma half-lives of the parent drug is ordinarily sufficient.
- Whole-body sections
- Section thickness normalized by quality control standards placed intro frozen blocks before sectioning
- 10-20 whole-body sections at various depths
- >40 tissues covered. Target tissue can be requested
- Blood sampling
- Tissue collection from frozen sample blocks after sectioning for QWBA
- Urine and feces
Endpoints
After at least four days of exposure, images are scanned and quantified relative to the calibration standards by image densitometry using appropriate image analysis software such as MCID. The drug concentrations derived from drug-related total radioactivity are used to generate a concentration-time profile, based on which tissue PK parameters are calculated using non-compartmental analysis. Following are general endpoints examined in a quantitative tissue distribution study and are customizable depending on your needs.
- Representative WBA images at each time point
- Concentrations of radioactivity in all significant tissues at each time point
- Tissue concentration versus time profiles
- Identified target tissues
Quotation and Ordering
As with all of our in vivo services, we ensure to keep in contact during studies. If you have any special needs or questions regarding our services, please feel free to contact us to support our experienced experts. We look forward to working with you in the future.
Reference
- FDA guidance for Industry. Safety Testing of Drug Metabolites. (March 2020)
Explore Other Options