Live Cell Imaging: Unveiling the Dynamic World of Cellular Processes
Live cell imaging has become an essential and powerful tool in cell research because it can provide dynamic information in real time and intuitively display the state and functional activities of cells under natural conditions. Compared with the common fixed-cell imaging technology, live cell imaging breaks the limitation of "snapshot" research and allows us to dynamically monitor and track cells, such as proliferation, migration, differentiation, and so on, by continuous observation. It will display the spatial and temporal dynamic regulatory networks of cells and bring new information and understanding for further study. In the cell-based assay, the combination of live cell imaging with fluorescence labeling technologies (fluorescent protein) further promotes the dynamic analysis of cells, and has become an indispensable part of drug screening, disease mechanism research and cell engineering. Not only in basic research but also in translational medicine scenarios such as preclinical drug development and personalized medicine, the application value of live cell imaging has been widely demonstrated.
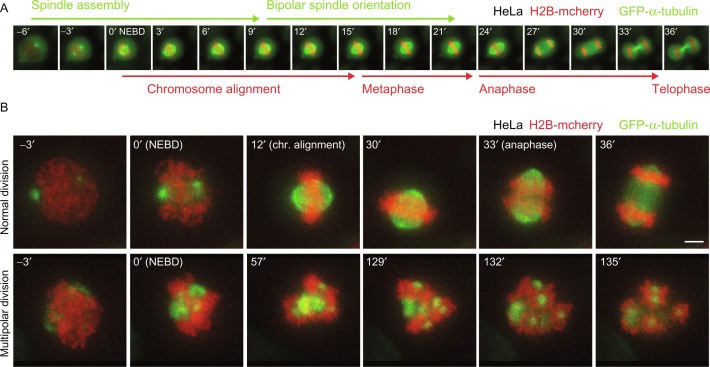 Fig. 1. Mitotic live-cell imaging at different timescales (Olziersky AM, Smith CA, et al. 2018).
Fig. 1. Mitotic live-cell imaging at different timescales (Olziersky AM, Smith CA, et al. 2018).
Overview of Live Cell Imaging Technology
Live cell imaging is a non-invasive technology to continuously monitor and record living cells in a physiologically relevant environment. The key principles of live cell imaging include:
- Fluorescence Labeling: Fluorescence labeling of target molecules (protein, nucleic acid, ion, etc.) with fluorescent proteins (GFP, RFP) or chemical fluorescent probes (calcium indicator Fluo-4), then excitation of the labeled molecules at specific wavelengths of light with fluorescence microscopy to image and record the real-time distribution and changes.
- Time-Resolved Imaging: Time-resolved imaging using high speed cameras and automated control software to continuously capture images at millisecond time resolution to record fast dynamic processes such as cytoskeletal rearrangement and vesicle trafficking.
- Environmental Control: Environmental control of the microscope system linked to an incubator to provide proper conditions for cell growth and maintenance (temperature, humidity, CO₂ concentration, etc.) so that experimental results are physiologically relevant.
Technical advantages
Live cell imaging has several technical advantages over conventional fixed-cell imaging:
- Real-Time and Dynamic: The method allows real-time, continuous and direct observation over hours to days, to monitor cellular behavior and dynamic events that are transient in nature, such as early signals during apoptosis which cannot be detected using conventional methods.
- Physiological Relevance: Since the cells are not subjected to fixation procedures that can cause structural damage to cells, their natural physiology is preserved.
- Multi-parameter Analysis: Multi-color fluorescence labeling can be used to monitor multiple molecules or signaling pathways simultaneously, e.g. simultaneous monitoring of calcium signaling and membrane potentials.
- High-Throughput: When combined with automated microscopy systems and image analysis software, the method allows high-throughput collection of dynamic data sets and quantitative analysis of large numbers of cells.
Applications of Live Cell Imaging in Cellular Assays
Cell Proliferation and Differentiation:
Labeling of cell cycle related proteins (e.g. PCNA, Cyclin) or nucleic acid dyes (e.g. EdU) using live cell imaging allows the tracking of individual cells in terms of their proliferation trajectory and population doubling times in real-time. The use of fluorescent reporter genes (e.g. Oct4-GFP) to label undifferentiated states in stem cells enables dynamic monitoring of differentiation into a specific lineage to reveal spatiotemporal expression patterns of key regulatory factors. Long-term imaging time courses (e.g. 72 hr continuous capture) also allows analysis of how proliferation rates interact with microenvironment conditions (e.g. cell density, matrix stiffness) which can provide data essential for tumor biology.
Cell Migration and Movement:
Cell migration is a fundamental process in many biological events, such as wound healing, embryonic development, and immune responses. Live cell imaging combined with scratch assays (Wound Healing Assay) or Transwell chambers can quantify cell migration speed, directionality, and group coordination. For example, fluorescently labeled actin (e.g., LifeAct-GFP) or focal adhesion proteins (e.g., Paxillin-RFP) enable real-time observation of pseudopodia formation, focal adhesion dynamic assembly, and cytoskeletal rearrangements, revealing the regulatory mechanisms of signaling pathways such as Rho GTPase in cell migration. Furthermore, three-dimensional live cell imaging techniques (e.g., confocal microscopy or light sheet microscopy) can create in vivo-like environments to study the infiltration behavior of cells in complex matrices.
Cell Signal Transduction:
Live cell imaging can be used to study the dynamic network of cell signal transduction. By labeling the important signaling molecules (such as calcium ions, cAMP, ROS) or transcription factors (such as NF-κB), the activation and transmission process of signal transduction can be observed in real time. For instance, by labeling calmodulin with FRET (Fluorescence Resonance Energy Transfer), the dynamic binding of calcium ions can be measured quantitatively to analyze the spatiotemporal dynamics of intracellular calcium signaling. By combining it with optogenetic tools (such as OptoGEF), specific signal transduction pathways can be activated, and their downstream effects can be studied with spatiotemporal resolution.
Cell Toxicity and Drug Screening:
In the process of drug development, live cell imaging is also widely used to study the drug's effect on cells. By using membrane potential-sensitive dyes (such as DiBAC₄(3)) or apoptosis markers (such as Caspase-3/7 activity fluorescent probes), the real-time occurrence of cell necrosis, apoptosis, or autophagy can be distinguished and quantitatively analyzed to establish the time-effect relationship of drug action. Furthermore, by integrating high-throughput microscopy with machine learning algorithms, the cellular morphological changes (such as nuclear shrinkage, membrane blebbing) can be automatically recognized, enabling the rapid screening and toxicity prediction of large compound libraries, which greatly improves the efficiency of drug development.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
| Cell-based Screening and Profiling Services | Speed up your drug discovery with Creative Bioarray's cell-based screening and profiling services. |
| Cell Line Testing and Assays | Creative Bioarray offers a wide range of cell line testing and assays from cell viability and proliferation to cellular phosphorylation assays. |
Reference
- Olziersky AM, Smith CA, et al. Mitotic live-cell imaging at different timescales. Methods Cell Biol. 2018. 145:1-27.