SLC Transporter Inhibition Assay
- Overview
- Services
- Features
- FAQ
- Explore Other Options
The Solute Carrier (SLC) transporter superfamily encompasses more than 400 membrane proteins that mediate the uptake and distribution of small molecules—including nutrients, metabolites, neurotransmitters, and pharmaceuticals—across critical biological barriers such as the liver, kidney, intestine, and blood–brain barrier. Several SLC transporters, including OATP1B1/1B3, OAT1/3, OCT1/2, and the MATE family, are of particular importance in drug development because of their central roles in hepatic and renal drug disposition and their potential to precipitate clinically relevant drug–drug interactions (DDIs).
- Regulatory Relevance: Regulatory agencies expect assessment of drug inhibition of SLC transporters to better understand DDIs.
- Clinical Impact: Inhibition of SLC transporters can result in altered drug exposure, toxicity, or decreased efficacy.
- Drug Development: Early screening and optimization of SLC transporters may inform the development of drug candidates and prevent late-stage failures.
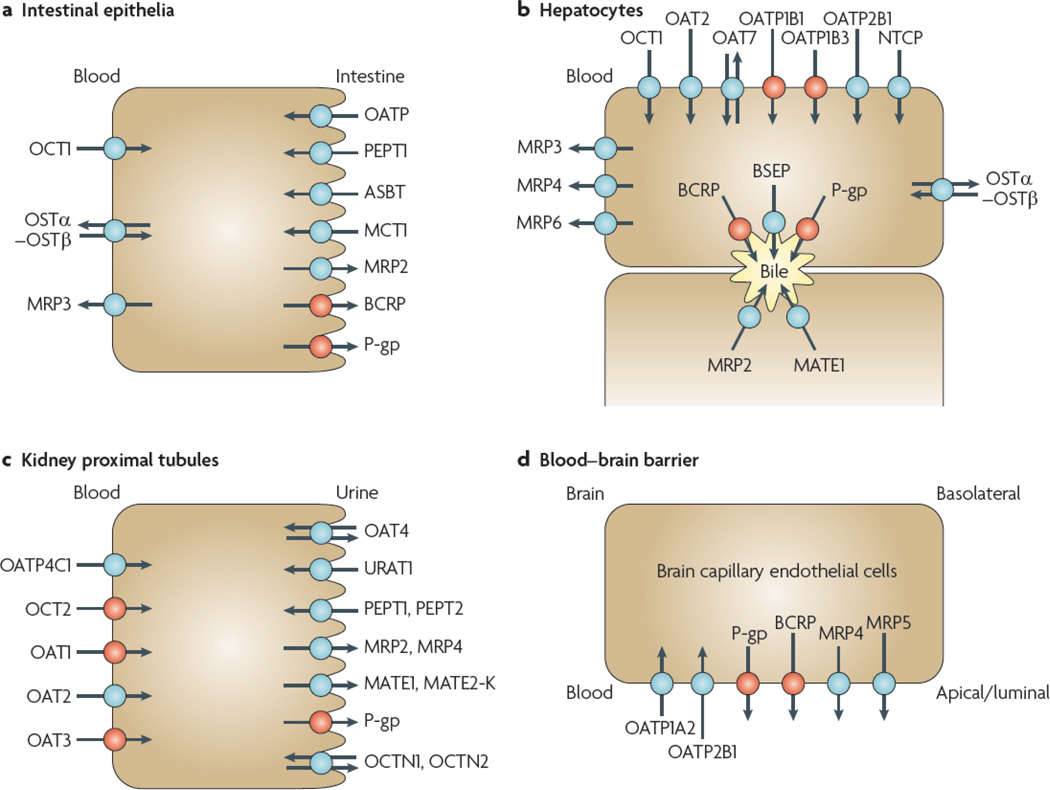 Fig. 1. Transporters in plasma membrane domains of intestinal epithelia, hepatocytes, kidney proximal tubules and brain capillary endothelial cells are presented (Giacomini KM, Huang SM, et al. 2010)
Fig. 1. Transporters in plasma membrane domains of intestinal epithelia, hepatocytes, kidney proximal tubules and brain capillary endothelial cells are presented (Giacomini KM, Huang SM, et al. 2010)
Service
Step 1: Cell Preparation
HEK-293 cells transiently overexpressing target SLC transporters (e.g., OATP1B1, OCT2) are thawed and cultured on poly-D-lysine–coated plates for 24 hours.
Step 2: Uptake Assay
cells are pre-incubated under optimized conditions (e.g., HBSS buffer, temperature control, substrate-specific treatments). Test compounds are added with or without inhibitors, and uptake is quantified following reaction termination.
Step 3: Quantification
Uptake is quantified by liquid scintillation counting and normalized to protein content.
Step 4: Data Analysis
Data analysis includes calculation of IC₅₀/Ki values and percent inhibition, with background subtraction from empty-vector controls.
Protocol
| Test System | HEK293 cells overexpressing human SLCs(OATP1B1、OATP1B3、OAT1、OAT3、OCT2、MATE1、MATE2-K、OCT1、PEPT1、PEPT2、OATP2B1—and other transporters available on request) |
| Test Article Concentration | Typically evaluated across 5–7 concentrations, optimized based on compound potency and toxicity data. |
| Time Point | Typically range from 1 to 10 minutes, depending on transporter kinetics. |
| Analysis Method | LC-MS/MS (high sensitivity) or radioactivity (high throughput) |
| Data Delivery | IC50 Ki values Inhibition curves |
| Probe Substrate | Estradiol 17-(β-D-glucuronide) (OATPB1, OATPB3) p-Aminohippurate (OAT1) Ranitidine (OAT3) TEA (OCT1) Metformin (OCT2, MATE1, MATE2-K) Glycyl sarcosine (PEPT1, PEPT2) Estrone 3-sulfate (OATP2B1) |
| Reference Inhibitor | Cycloporine (OATPB1, OATPB3) Probenecid (OAT1, OAT3) Verapamil (OCT1, OCT2) Pyrimethamine (MATE1, MATE2-K) Losartan (PEPT1, PEPT2) |
Features
Comprehensive Coverage & Custom Flexibility
SLC transporters supported include OATP, OAT, OCT, MATE, PEPT, NTCP, and more. We offer transporter panels and assay design customizations to fit your study needs.
Optional Pre-incubation for Enhanced Reliability
SLC transport assays with this service offer flexible pre-incubation protocols to account for the substantial impact pre-incubation can have on IC50 determinations.
Multi-Platform Detection Solutions
Our portfolio of detection platforms, including the high sensitivity LC-MS/MS quantification and higher throughput fluorescence/radioactive detection, is tailored for every research stage.

Expert Support
Custom study designs and data interpretation from experienced pharmacologists.
FAQ
Why is SLC transporter inhibition testing important?
Inhibition of SLC transporters can lead to clinically significant DDIs, affecting drug safety and efficacy. Regulatory agencies require these studies for new drug applications.
Can you support high-throughput screening (HTS)?
Yes, we offer 96/384-well plate formats for early-stage screening of large compound libraries.
How should I choose between fluorescence- and radioactivity-based assays?
The fluorescence method offers non-radioactive detection and simpler workflows, making it ideal for high-throughput screening. The radioactive isotope method provides higher sensitivity, recommended for low-abundance targets or weak-affinity compounds.
How do you ensure data reliability?
We use validated methods, strict QC criteria, and include positive/negative controls in every assay.
Reference
- Giacomini KM, Huang SM, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010. 9(3):215-36.
Explore Other Options