Chemical Stability Assays
- Service Details
- Features
- Explore Other Options
Chemical stability plays a pivotal role in determining a drug's safety, efficacy, and shelf life throughout its development and commercialization. At Creative Bioarray, we provide customized Chemical Stability Assays to characterize the degradation behavior of drug substances and formulations under various environmental and physiological conditions. Our goal is to help clients understand how their compounds withstand chemical stress, guiding formulation strategies, packaging choices, and regulatory submissions.
Why Chemical Stability Testing Matters
Chemical stability refers to a drug's ability to maintain its identity, potency, and purity throughout the manufacturing process, storage, and administration to patients. If a drug is not chemically stable, it may lose its therapeutic activity, produce toxic degradation products, or have altered bioavailability.
- Hydrolysis and dehydration in aqueous environments
- Oxidation triggered by oxygen or trace metals
- Photolytic reactions induced by light exposure
- Interactions with excipients or container materials
- Evaluate compound suitability for oral or parenteral administration
- Inform formulation design and excipient selection
- Establish optimal packaging and storage conditions
- Support regulatory documentation in compliance with ICH Q1A(R2) and related guidelines
Our Service
We evaluate the inherent stability of drug substances and products using physiologically relevant media—such as Simulated Gastric Fluid (SGF), Simulated Intestinal Fluid (SIF), and buffers across a physiological pH range—coupled with advanced analytical techniques like LC-MS/MS and HPLC.
Workflow
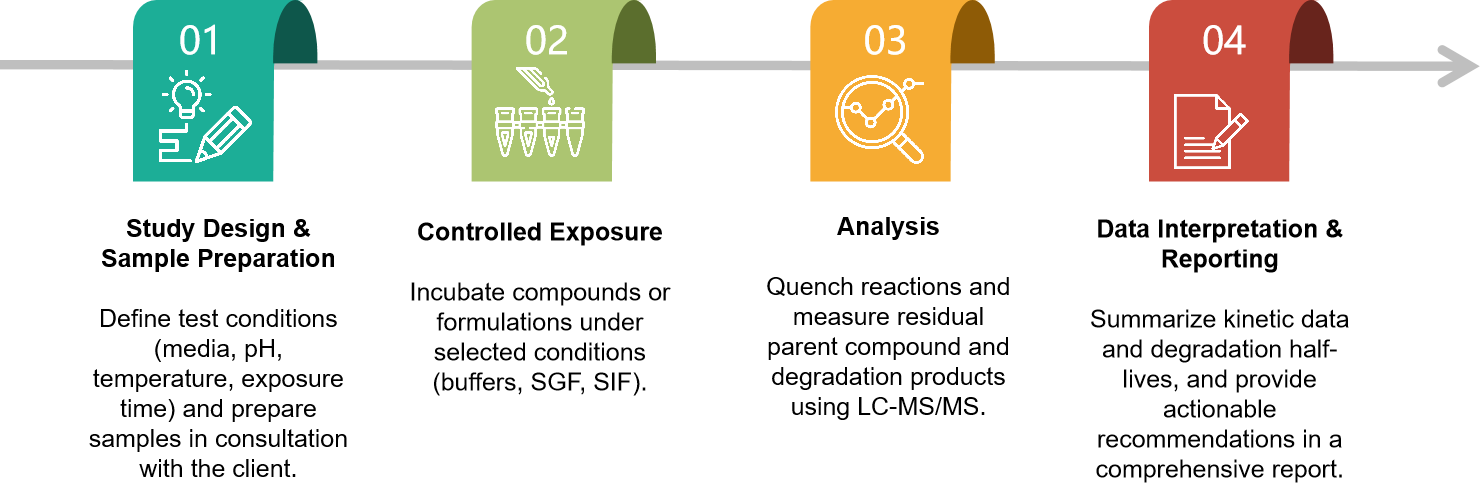
Protocol
| Parameter | Typical/Recommended Condition |
| Test Media | Buffer Systems: pH (1.0 – 8.0) Simulated Gastric Fluid (SGF): pH 1.2 (with/without pepsin) Simulated Intestinal Fluid (SIF): pH 6.8 (with/without pepsin) |
| Time Points | 0, 15, 30, 60, 120, 240 min |
| Test Article Concentration | 1-5μM (different concentrations available) |
| Analytical Method | LC-MS/MS, HPLC |
| Format | 96-well format, shaking incubator at chosen temperature. |
| Positive controls | Eucatropine, Benfluorex, Chlorambucil or Omeprazole |
| Data Delivery | % Parent compound remaining at each time point Half Life |
Why Choose Us

Scientific Expertise
Extensive experience in small-molecule and peptide stability testing.
Custom Flexibility
Tailored assay conditions for early discovery screens or formal stability studies.

Advanced Instrumentation
State-of-the-art analytical platforms for precise and reproducible results.
Actionable Insights
Beyond data reporting, we provide interpretation and practical recommendations to mitigate stability issues.
Explore Other Options