GMP iPSC & MSC Manufacturing
- Service Details
- Features
- FAQ
- Explore Other Options
Cell therapy, as a cutting-edge medical technology, is bringing new hope to various refractory diseases. Induced pluripotent stem cells (iPSCs) and mesenchymal stem cells (MSCs) represent the two primary cell types in cell therapy research. iPSCs serve as key tools in regenerative medicine and disease modeling because they can proliferate indefinitely and differentiate into various cell types while MSCs are recognized for their abilities to regulate immune responses and repair tissues. With the continuous advancement of cell therapy technologies, there is an increasing demand for the quality and safety of iPSCs and MSCs.
Our GMP iPSC & MSC manufacturing services deliver a comprehensive solution that enables them navigate through all stages from basic research to commercial-scale production. Leveraging our specialized technical team, state-of-the-art GMP-compliant facilities, and extensive production experience, we are dedicated to offering efficient and reliable customized cell manufacturing services for cell therapy projects worldwide, assisting clients in accelerating the market launch of their products and promoting the widespread application of cell therapy technologies.
Custom GMP iPSC & MSC Manufacturing Services
Donor and Sample Management
We collaborate with globally compliant donor banks to offer diverse sources (such as peripheral blood, umbilical cord, and fibroblasts) for sample collection, screening, and ethical compliance support.
iPSC Full-Process Services
- Utilizing mRNA-LNP non-viral reprogramming technology for the efficient and rapid generation of iPSCs without the risk of gene integration.
- Gene editing, supporting gene correction, knock-in/out, and reporter gene construction.
- Monoclonal screening, Master Cell Bank (MCB) construction, and genetic stability validation.
MSC Scale-Up Production
- Optimization of tissue dissociation, adherent expansion, and serum-free culture processes to ensure high activity and phenotypic stability.
- Establishment and comprehensive quality control of cell banks (working and master banks), including immunophenotyping, differentiation potential, and karyotype analysis.
Analysis and Quality Control
- Release Testing: sterility, mycoplasma, endotoxin, cell viability, identity verification (STR analysis), residual DNA/RNA detection.
- Functional Validation: differentiation capability, secreted factor profile, immunomodulatory activity.
Workflow
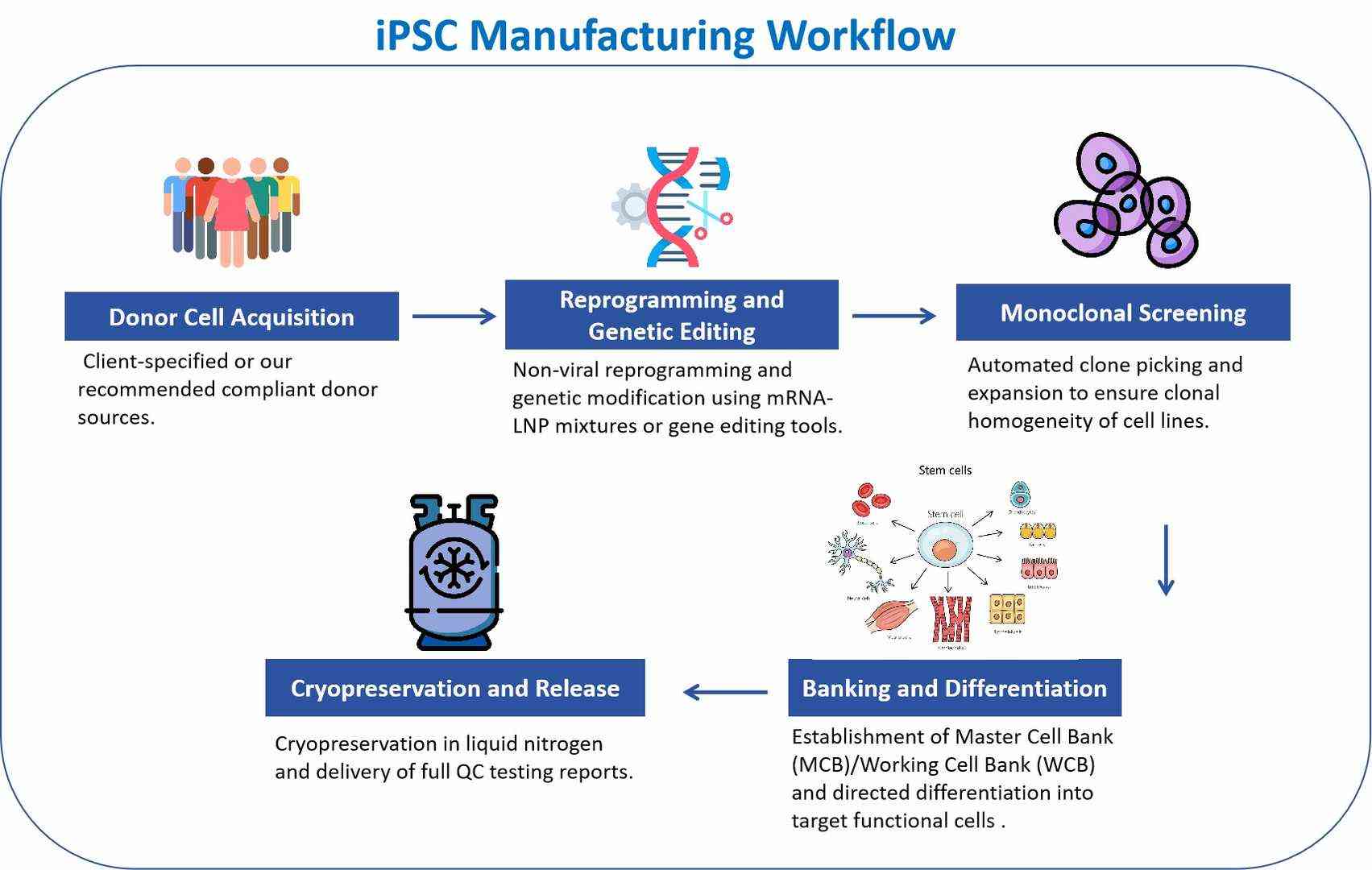
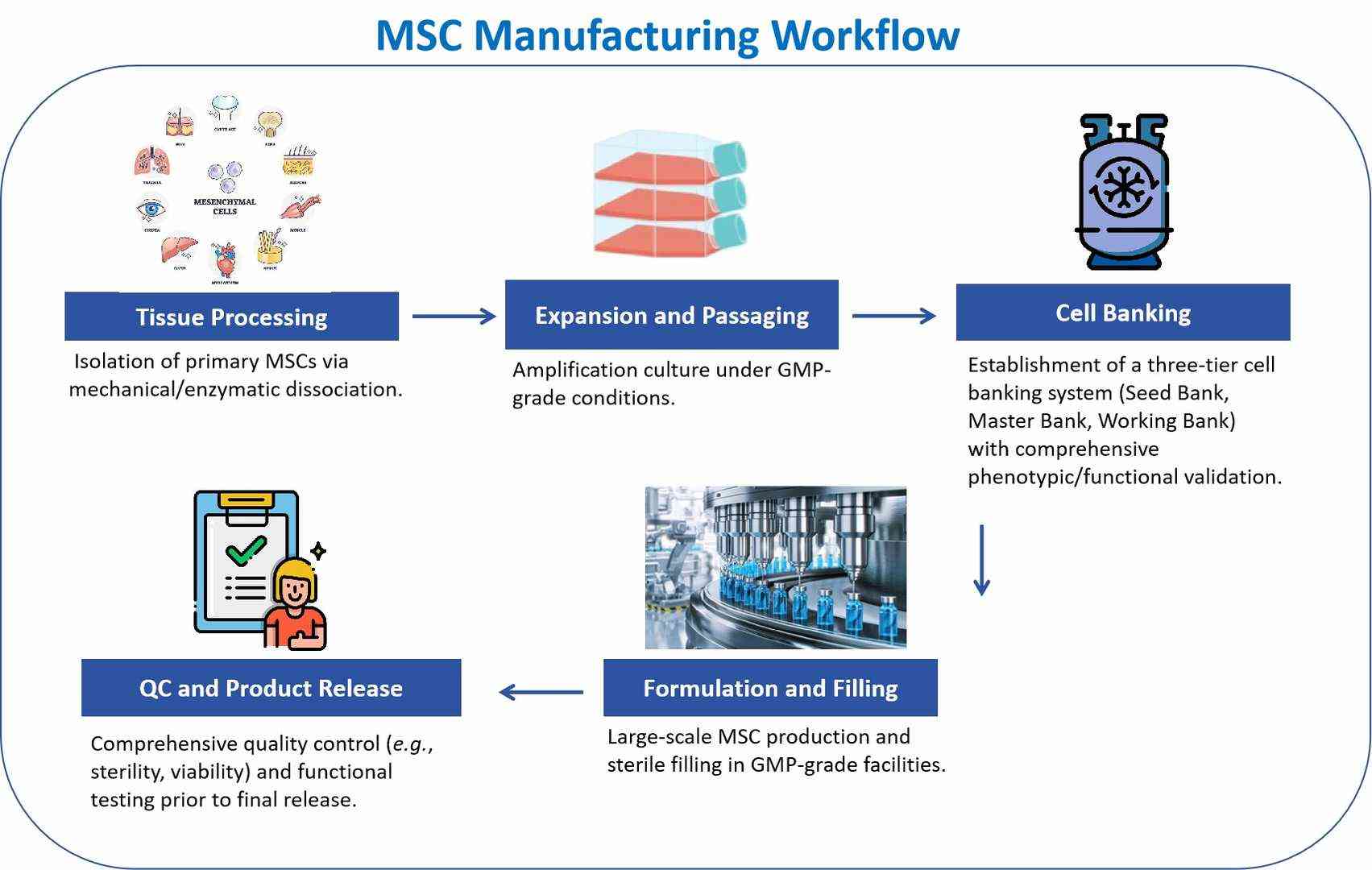
Features

Global Compliance Facilities
The GMP workshop complies with FDA and EMA standards, equipped with isolator systems and real-time environmental monitoring.
Extensive Production Experience
Successful production of multiple batches of GMP-grade iPSCs and MSCs. Fully compliant with regulatory guidelines and GMP standards.
Innovative iPSC reprogramming technology
Efficient iPSC reprogramming using mRNA-LNP mixtures, ensuring high generation efficiency.

Streamlined Production Process
One-stop services from cell collection to product release, ensuring quality and reproducibility.
FAQ
1. Do you support customized gene editing?
Yes, we offer one-stop services for target design, vector construction, and editing efficiency verification.
2. Does iPSC reprogramming lead to genetic abnormalities?
Our mRNA-LNP technology eliminates the need for viral vectors and we maintain genetic stability by performing whole genome sequencing (WGS) and karyotype analysis according to ICH Q5A requirements.
3. How do you ensure the quality and stability of the products?
Our strict quality control system covers the entire production journey from acquiring raw materials through production process management and extends to final product release. Each production step undergoes thorough quality testing that covers cell identification, purity assessment, sterility examination, viability tests along with genetic stability evaluation. Additionally, our advanced production technologies and equipment enable real-time monitoring and adjustment of the production process to maintain product quality and stability.
4. How do you ensure the quality and safety of donor cells?
In partnership with certified service providers, we implement strict adherence to relevant standards for donor screening and sample collection. The screening procedure includes thorough evaluations of both the donor's health status and genetic background to guarantee donor cells remain free from infectious diseases and genetic defects. The sample collection, transportation, and storage processes implement stringent measures that protect cell viability and safety.
Explore Other Options