3D-Cell Model in Cell-Based Assay
2D cell models are grown on a flat surface in a single layer. These models are inexpensive and simple to use for large-scale screening. However, they do not accurately reflect in vivo tissue conditions. The lack of 3D interactions and nutrient diffusion can lead to abnormal cell behavior.
3D cell models more closely resemble in vivo tissues. They support complex interactions, more accurately predict drug screenings, and can be applied to cell therapies. These models are more complex, expensive, and difficult to apply to high-throughput screenings. They also require specialized methods for analysis. The choice of cell model depends on the experimental goals and resources available.
Table 1. Advantages and limitations of culture models 'at a glance' (Blasio, S. Di, 2017).
| Culture model | Advantages | Limitations |
| 2D |
|
|
| 3D |
|
|
Types of 3D Cell Models:
3D cell models come in various types, each with unique features and applications. Here are some main types:
- Scaffold-free Models: These do not require external scaffold support, allowing cells to naturally form 3D structures. Examples of scaffold-free 3D cell culture models include hanging drop culture, ultra-low attachment plates, and magnetic levitation. Cells are cultured without attachment, enabling free movement and interaction in the three-dimensional space.
- Scaffold-based Models: Scaffold-based 3D cell models provide biocompatible support structures or matrices for cells to grow in three-dimensional structures. The scaffolds may be of natural origin (such as collagen gels, decellularized matrix gels, or hydrogels), or synthetically made (polymeric hard scaffolds and hydrogels). Scaffolds provide a stable environment for cell growth, making it ideal for tissue engineering and regenerative medicine applications.
- Microfluidics and Organ-on-a-chip: Microfluidic technology refers to combining microfluidic systems and cell culture to simulate the microenvironment of organs on a small chip. The advantage of organ-on-a-chip is that it can precisely control the conditions of the organ growth microenvironment, support the co-culture of multiple organs, and accurately mimic the in vivo microenvironment and structure. The application value of organ-on-a-chip in drug screening and disease research is becoming more and more prominent.
- 3D Bioprinting: 3D bioprinting constructs three-dimensional cell structures by layer-by-layer addition of bio-inks and bio-gels. This method achieves high cell survival rates and densities, suitable for tissue engineering, regenerative medicine, and organ printing. The major advantage of bioprinting technology is the precise control of cell distribution and arrangement, which can construct more complex tissue structures.
- Organoids: Organoids are highly simplified organ models that mimic the microanatomy and function of specific organs. Usually derived from stem cells, organoids form 3D structures in vitro with specific cell types and tissue structures. They have broad applications in human disease modeling, drug screening, and personalized medicine.
The selection of suitable models depends on research needs because each 3D cell model offers distinct benefits and limitations. Scaffold-free models are suitable for studying the natural interaction of cells. In contrast, scaffold-based models are used for cell culture that requires a stable scaffold. Microfluidics and organ-on-a-chip are used for simulating more complex microenvironments, and bioprinting and organoids are used in tissue engineering and regenerative medicine.
Applications of 3D Cell Models in Cell-Based Assays:
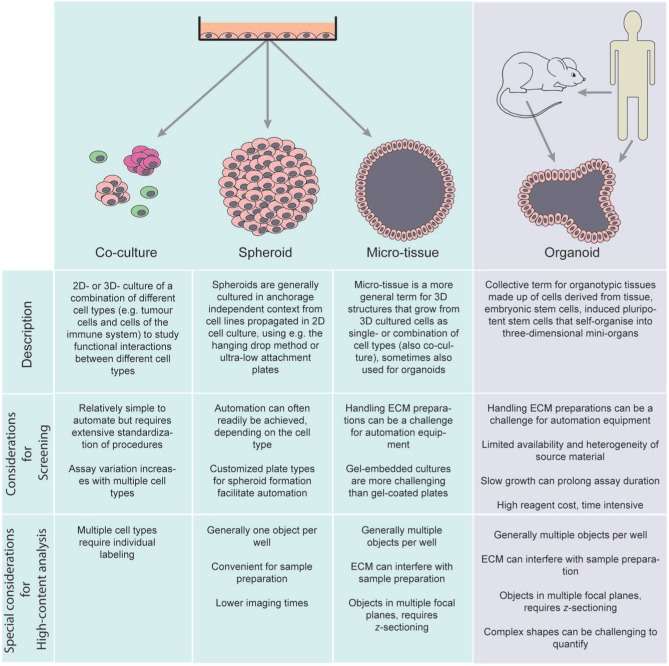 Fig. 1. Nomenclature of 3D cell-based assays (Booij TH, Price LS, et al. 2019).
Fig. 1. Nomenclature of 3D cell-based assays (Booij TH, Price LS, et al. 2019).
Drug development and toxicity testing:
- Anti-Tumor Drug Screening: 3D tumor spheroid models reveal resistance mechanisms: EGFR inhibitors display IC50 values 10 times higher in 3D than in 2D due to hypoxic regions in 3D structures inducing HIF-1α expression, activating the PI3K/AKT alternative pathway.
- Cardiotoxicity Assessment: Cardiac organoids (with electrical signal conduction functions) detect drug-induced arrhythmia risks: By 2025, EMA guidelines will require all new drugs to undergo QT interval extension tests using 3D cardiac chips.
- Hepatotoxicity Prediction: 3D hepatocyte spheroids have 4.7 times increased metabolic activity, accurately reflecting drug metabolism enzymes (e.g., CYP3A4) induction effects: Rifampicin induces CYP3A4 expression 6 times more in 3D models than in 2D.
Disease mechanisms research:
- Cancer Biology: 3D models simulate the tumor microenvironment: In 3D breast tumor models, cancer-associated fibroblasts (CAFs) promote tumor cell epithelial-mesenchymal transition (EMT) by secreting IL-6, an effect suppressed in 2D models.
- Neurodegenerative Diseases: 3D brain organoids replicate tau protein aggregation: In Alzheimer's organoids, tau protein phosphorylation levels reach 80% of patient brain tissue levels in 6 months, forming neurofibrillary tangles (NFTs).
- Rare Disease Modeling: Patient-derived iPSCs construct 3D models: In cystic fibrosis lung organoids, CFTR protein dysfunction leads to mucus secretion abnormalities, with chloride channel activity restored by 65% after Ivacaftor treatment.
Tissue engineering and regenerative medicine:
- Skin Repair: 3D skin models (including keratinocytes and fibroblasts) used for burn treatment product testing: The 2024 FDA-approved ReCell® autologous cell regeneration technology, based on 3D skin models, optimizes cell spray density, reducing wound healing time by 30%.
- Bone Regeneration: 3D printed scaffolds combined with MSCs differentiate into osteoblasts: In a rabbit femoral defect model, nano-hydroxyapatite/polycaprolactone (nHA/PCL) scaffolds achieve an 85% bone regeneration rate, significantly higher than traditional titanium implants (60%).
Technical Challenges and Solutions of 3D Cell Models in Cell-based Assay
Although 3D cell models have several advantages in drug screening and disease research, there are still some challenges:
- Low Experimental Throughput: 3D cell models are more complex to culture and assay than 2D, leading to lower throughput.
- Difficulty of imaging and analysis: 3D cell models require more advanced microscopy techniques and image processing algorithms for accurate and reproducible data acquisition and analysis.
- High cost: The cost of culture medium, reagents, and equipment for 3D cell models is generally higher than for 2D cell models, which is not conducive to the promotion of 3D cell models in large-scale drug screening applications.
- Lack of Standardization and Validation: Currently, there is a lack of unified standardization and validation methods for 3D models, which limits the application of 3D models in industry.
Solutions:
- Optimize Detection Methods: Develop assays specifically targeting 3D cell models.
- Improve imaging technology: High-content analysis (HCA) technology can simultaneously detect multiple parameters in cells and provide more comprehensive and accurate drug screening information, which can improve the efficiency and accuracy of drug screening. Microfluidic systems can also improve the quality of imaging and analysis of 3D cell models and improve the efficiency of data analysis.
- Promote Standardization and Automation: Standardization and automation can improve the reproducibility and high-throughput screening capabilities of 3D cell models. In addition, more efficient 3D cell culture technologies are also being developed, such as 3D bioprinting, which can precisely arrange cells and construct tissue structures.
Creative Bioarray Relevant Recommendations
| Products & Services | Description |
| Cell-based Screening and Profiling Services | Speed up your drug discovery with Creative Bioarray's cell-based screening and profiling services. |
| 3D-based Services | Creative Bioarray conducts the custom 3D-based cell services with the comprehensive and advanced 3D culture systems in the field to ensure the best result for your research goals |
Reference
- Booij TH, Price LS, et al. 3D Cell-Based Assays for Drug Screens: Challenges in Imaging, Image Analysis, and High-Content Analysis. SLAS Discov. 2019 Jul;24(6):615-627.